MiR-194, commonly repressed in colorectal cancer, suppresses tumor growth by regulating the MAP4K4/c-Jun/MDM2 signaling pathway
- PMID: 25602366
- PMCID: PMC4615039
- DOI: 10.1080/15384101.2015.1007767
MiR-194, commonly repressed in colorectal cancer, suppresses tumor growth by regulating the MAP4K4/c-Jun/MDM2 signaling pathway
Abstract
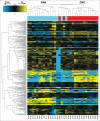
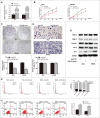
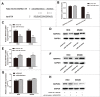
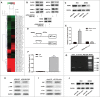
Tumor growth cascade is a complicated and multistep process with numerous obstacles. Until recently, evidences have shown the involvement of microRNAs (miRNAs) in tumorigenesis and tumor progression of various cancers, including colorectal cancer (CRC). In this study, we explored the role of miR-194 and its downstream pathway in CRC. We acquired data through miRNA microarray profiles, showing that the expression of miR-194 was significantly suppressed in CRC tissues compared with corresponding noncancerous tissues. Decreased miR-194 expression was obviously associated with tumor size and tumor differentiation, as well as TNM stage. Both Kaplan-Meier and multivariate survival analysis showed that downregulated miR-194 was associated with overall survival. Moreover, functional assays indicated that overexpression of miR-194 in CRC cell lines inhibited cell proliferation both in vitro and in vivo. In addition, using dual-luciferase reporter gene assay, we found MAP4K4 was the direct target of miR-194. Silencing of MAP4K4 resulted in similar biological behavior changes to that of overexpression of miR-194. We also observed through Human Gene Expression Array that MDM2 was one of the downstream targets of MAP4K4. Knockdown of MAP4K4 downregulated MDM2 expression through transcription factor c-Jun binding to the -1063 to -1057 bp of the promoter. These results suggest that miR-194, regulating the MAP4K4/c-Jun/MDM2 signaling pathway, might act as a tumor suppressor and serve as a novel target for CRC prevention and therapy.
Keywords: MAP4K4; MDM2; apoptosis; c-Jun; colorectal cancer; miR-194; proliferation.
Figures


References
-
- Siegel R, Desantis C, Jemal A. Colorectal cancer statistics, 2014. CA Cancer J Clin 2014; 64:104-17; PMID:24639052; http://dx.doi.org/10.3322/caac.21220 - DOI - PubMed
-
- Chen DL, Wang ZQ, Zeng ZL, Wu WJ, Zhang DS, Luo HY, Wang F, Qiu MZ, Wang DS, Ren C, et al. . Identification of MicroRNA-214 as a negative regulator of colorectal cancer liver metastasis by way of regulation of fibroblast growth factor receptor 1 expression. Hepatology 2014; 60:598-609; PMID:24616020; http://dx.doi.org/10.1002/hep.27118 - DOI - PubMed
-
- Valeri N, Braconi C, Gasparini P, Murgia C, Lampis A, Paulus-Hock V, Hart JR, Ueno L, Grivennikov SI, Lovat F, et al. . MicroRNA-135b promotes cancer progression by acting as a downstream effector of oncogenic pathways in colon cancer. Cancer Cell 2014; 25:469-83; PMID:24735923; http://dx.doi.org/10.1016/j.ccr.2014.03.006 - DOI - PMC - PubMed
-
- Sjoblom T, Jones S, Wood LD, Parsons DW, Lin J, Barber TD, Mandelker D, Leary RJ, Ptak J, Silliman N, et al. . The consensus coding sequences of human breast and colorectal cancers. Science 2006; 314:268-74; PMID:16959974; http://dx.doi.org/10.1126/science.1133427 - DOI - PubMed
-
- De Roock W, De Vriendt V, Normanno N, Ciardiello F, Tejpar S. KRAS, BRAF, PIK3CA, and PTEN mutations: implications for targeted therapies in metastatic colorectal cancer. Lancet Oncol 2011; 12:594-603; PMID:21163703; http://dx.doi.org/10.1016/S1470-2045(10)70209-6 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous
