Ligand-induced dynamics of neurotrophin receptors investigated by single-molecule imaging approaches
- PMID: 25603178
- PMCID: PMC4307343
- DOI: 10.3390/ijms16011949
Ligand-induced dynamics of neurotrophin receptors investigated by single-molecule imaging approaches
Abstract
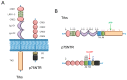
Neurotrophins are secreted proteins that regulate neuronal development and survival, as well as maintenance and plasticity of the adult nervous system. The biological activity of neurotrophins stems from their binding to two membrane receptor types, the tropomyosin receptor kinase and the p75 neurotrophin receptors (NRs). The intracellular signalling cascades thereby activated have been extensively investigated. Nevertheless, a comprehensive description of the ligand-induced nanoscale details of NRs dynamics and interactions spanning from the initial lateral movements triggered at the plasma membrane to the internalization and transport processes is still missing. Recent advances in high spatio-temporal resolution imaging techniques have yielded new insight on the dynamics of NRs upon ligand binding. Here we discuss requirements, potential and practical implementation of these novel approaches for the study of neurotrophin trafficking and signalling, in the framework of current knowledge available also for other ligand-receptor systems. We shall especially highlight the correlation between the receptor dynamics activated by different neurotrophins and the respective signalling outcome, as recently revealed by single-molecule tracking of NRs in living neuronal cells.
Figures

Similar articles
-
Small-molecule modulation of neurotrophin receptors: a strategy for the treatment of neurological disease.Nat Rev Drug Discov. 2013 Jul;12(7):507-25. doi: 10.1038/nrd4024. Nat Rev Drug Discov. 2013. PMID: 23977697 Review.
-
Local versus long-range neurotrophin receptor signalling: endosomes are not just carriers for axonal transport.Semin Cell Dev Biol. 2014 Jul;31:57-63. doi: 10.1016/j.semcdb.2014.03.032. Epub 2014 Apr 5. Semin Cell Dev Biol. 2014. PMID: 24709025 Review.
-
Proteolytic processing of the p75 neurotrophin receptor: A prerequisite for signalling?: Neuronal life, growth and death signalling are crucially regulated by intra-membrane proteolysis and trafficking of p75(NTR).Bioessays. 2011 Aug;33(8):614-25. doi: 10.1002/bies.201100036. Epub 2011 Jun 30. Bioessays. 2011. PMID: 21717487 Review.
-
Spatiotemporal intracellular dynamics of neurotrophin and its receptors. Implications for neurotrophin signaling and neuronal function.Handb Exp Pharmacol. 2014;220:33-65. doi: 10.1007/978-3-642-45106-5_3. Handb Exp Pharmacol. 2014. PMID: 24668469 Review.
-
Truncated TrkB receptor-induced outgrowth of dendritic filopodia involves the p75 neurotrophin receptor.J Cell Sci. 2004 Nov 15;117(Pt 24):5803-14. doi: 10.1242/jcs.01511. Epub 2004 Oct 26. J Cell Sci. 2004. PMID: 15507485
Cited by
-
Fluorolabeling of the PPTase-Related Chemical Tags: Comparative Study of Different Membrane Receptors and Different Fluorophores in the Labeling Reactions.Front Mol Biosci. 2020 Aug 7;7:195. doi: 10.3389/fmolb.2020.00195. eCollection 2020. Front Mol Biosci. 2020. PMID: 32850976 Free PMC article.
-
Lysosome Dynamic Properties during Neuronal Stem Cell Differentiation Studied by Spatiotemporal Fluctuation Spectroscopy and Organelle Tracking.Int J Mol Sci. 2020 May 11;21(9):3397. doi: 10.3390/ijms21093397. Int J Mol Sci. 2020. PMID: 32403391 Free PMC article.
-
p75 Neurotrophin Receptor in the Skin: Beyond Its Neurotrophic Function.Front Med (Lausanne). 2017 Mar 7;4:22. doi: 10.3389/fmed.2017.00022. eCollection 2017. Front Med (Lausanne). 2017. PMID: 28326307 Free PMC article. Review.
-
Tropomyosin receptor kinase B-mediated signaling in integration of neuropathic pain and obesity in diabetic polyneuropathy.Einstein (Sao Paulo). 2021 Sep 27;19:eAO6256. doi: 10.31744/einstein_journal/2021AO6256. eCollection 2021. Einstein (Sao Paulo). 2021. PMID: 34586159 Free PMC article.
-
Trajectory Analysis in Single-Particle Tracking: From Mean Squared Displacement to Machine Learning Approaches.Int J Mol Sci. 2024 Aug 8;25(16):8660. doi: 10.3390/ijms25168660. Int J Mol Sci. 2024. PMID: 39201346 Free PMC article. Review.
References
-
- Lewin G.R., Carter B.D. Neurotrophic Factors. Springer; Berlin-Heidelberg, Germany: 2014.
-
- Skaper S.D. Neuarotrophic Factors: Methods and Protocols. Humana Press; Totowa, NJ, USA: 2012.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

