The role of reactive oxygen species in mesenchymal stem cell adipogenic and osteogenic differentiation: a review
- PMID: 25603196
- PMCID: PMC4424969
- DOI: 10.1089/scd.2014.0484
The role of reactive oxygen species in mesenchymal stem cell adipogenic and osteogenic differentiation: a review
Abstract
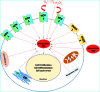
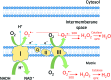
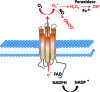
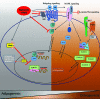
Mesenchymal stromal cells (MSCs) are promising candidates for tissue engineering and regenerative medicine. The multipotent stem cell component of MSC isolates is able to differentiate into derivatives of the mesodermal lineage including adipocytes, osteocytes, chondrocytes, and myocytes. Many common pathways have been described in the regulation of adipogenesis and osteogenesis. However, stimulation of osteogenesis appears to suppress adipogenesis and vice-versa. Increasing evidence implicates a tight regulation of these processes by reactive oxygen species (ROS). ROS are short-lived oxygen-containing molecules that display high chemical reactivity toward DNA, RNA, proteins, and lipids. Mitochondrial complexes I and III, and the NADPH oxidase isoform NOX4 are major sources of ROS production during MSC differentiation. ROS are thought to interact with several pathways that affect the transcription machinery required for MSC differentiation including the Wnt, Hedgehog, and FOXO signaling cascades. On the other hand, elevated levels of ROS, defined as oxidative stress, lead to arrest of the MSC cell cycle and apoptosis. Tightly regulated levels of ROS are therefore critical for MSC terminal differentiation, although the precise sources, localization, levels and the exact species of ROS implicated remain to be determined. This review provides a detailed overview of the influence of ROS on adipogenic and osteogenic differentiation in MSCs.
Figures

References
-
- Harman D. (1956). Aging: a theory based on free radical and radiation chemistry. J Gerontol 11:298–300 - PubMed
-
- Beckman KB. and Ames BN. (1998). The free radical theory of aging matures. Physiol Rev 78:547–581 - PubMed
-
- Guzik TJ. and Harrison DG. (2006). Vascular NADPH oxidases as drug targets for novel antioxidant strategies. Drug Discov Today 11:524–533 - PubMed
-
- Coso S, Harrison I, Harrison CB, Vinh A, Sobey CG, Drummond GR, Williams ED. and Selemidis S. (2012). NADPH oxidases as regulators of tumor angiogenesis: current and emerging concepts. Antioxid Redox Signal 16:1229–1247 - PubMed
-
- Bedard K. and Krause KH. (2007). The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol Rev 87:245–313 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
