Development of highly selective Kv1.3-blocking peptides based on the sea anemone peptide ShK
- PMID: 25603346
- PMCID: PMC4306950
- DOI: 10.3390/md13010529
Development of highly selective Kv1.3-blocking peptides based on the sea anemone peptide ShK
Abstract
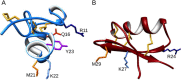
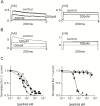
ShK, from the sea anemone Stichodactyla helianthus, is a 35-residue disulfide-rich peptide that blocks the voltage-gated potassium channel Kv1.3 at ca. 10 pM and the related channel Kv1.1 at ca. 16 pM. We developed an analog of this peptide, ShK-186, which is currently in Phase 1b-2a clinical trials for the treatment of autoimmune diseases such as multiple sclerosis and rheumatoid arthritis. While ShK-186 displays a >100-fold improvement in selectivity for Kv1.3 over Kv1.1 compared with ShK, there is considerable interest in developing peptides with an even greater selectivity ratio. In this report, we describe several variants of ShK that incorporate p-phophono-phenylalanine at the N-terminus coupled with internal substitutions at Gln16 and Met21. In addition, we also explored the combinatorial effects of these internal substitutions with an alanine extension at the C-terminus. Their selectivity was determined by patch-clamp electrophysiology on Kv1.3 and Kv1.1 channels stably expressed in mouse fibroblasts. The peptides with an alanine extension blocked Kv1.3 at low pM concentrations and exhibited up to 2250-fold selectivity for Kv1.3 over Kv1.1. Analogs that incorporates p-phosphono-phenylalanine at the N-terminus blocked Kv1.3 with IC50s in the low pM range and did not affect Kv1.1 at concentrations up to 100 nM, displaying a selectivity enhancement of >10,000-fold for Kv1.3 over Kv1.1. Other potentially important Kv channels such as Kv1.4 and Kv1.6 were only partially blocked at 100 nM concentrations of each of the ShK analogs.
Figures



References
-
- Pennington M.W., Kem W.R., Karlsson E. Sea anemone potassium channel toxins. In: Montecucco C.A.R., editor. Guidebook to Protein Toxins and Their Use in Cell Biology. Oxford University Press; Oxford, UK: 1997. pp. 159–161.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

