Regulation of transcription by eukaryotic-like serine-threonine kinases and phosphatases in Gram-positive bacterial pathogens
- PMID: 25603430
- PMCID: PMC4601284
- DOI: 10.4161/21505594.2014.983404
Regulation of transcription by eukaryotic-like serine-threonine kinases and phosphatases in Gram-positive bacterial pathogens
Abstract
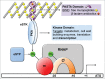
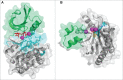
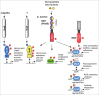
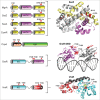
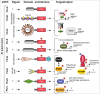
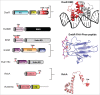
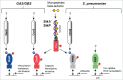
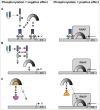
Bacterial eukaryotic-like serine threonine kinases (eSTKs) and serine threonine phosphatases (eSTPs) have emerged as important signaling elements that are indispensable for pathogenesis. Differing considerably from their histidine kinase counterparts, few eSTK genes are encoded within the average bacterial genome, and their targets are pleiotropic in nature instead of exclusive. The growing list of important eSTK/P substrates includes proteins involved in translation, cell division, peptidoglycan synthesis, antibiotic tolerance, resistance to innate immunity and control of virulence factors. Recently it has come to light that eSTK/Ps also directly modulate transcriptional machinery in many microbial pathogens. This novel form of regulation is now emerging as an additional means by which bacteria can alter their transcriptomes in response to host-specific environmental stimuli. Here we focus on the ability of eSTKs and eSTPs in Gram-positive bacterial pathogens to directly modulate transcription, the known mechanistic outcomes of these modifications, and their roles as an added layer of complexity in controlling targeted RNA synthesis to enhance virulence potential.
Keywords: OCS, one-component signaling; PASTA, penicillin-binding protein and Ser/Thr kinase associated; PPM, protein phosphatase metal binding; PTM, posttranslational modification; REC, receiver; ROS, reactive oxygen species; TCS, two-component signaling; bacteria; eSTK, eukaryotic-like serine-threonine kinase; eSTP, eukaryotic-like serine-threonine phosphatase; infection; phosphorylation; serine threonine kinase; serine threonine phosphatase; transcription; wHTH, winged helix-turn-helix.
Figures


References
-
- Hoch JA, Silhavy TJ, eds. Two-component signal transduction. Washington, D.C.: American Society for Microbiology, 1995
-
- Stock JB, Stock AM, Mottonen JM. Signal transduction in bacteria. Nature 1990; 344:395-400; PMID:2157156; http://dx.doi.org/10.1038/344395a0 - DOI - PubMed
-
- Stock AM, Robinson VL, Goudreau PN. Two-component signal transduction. Annu Rev Biochem 2000; 69:183-215; PMID:10966457; http://dx.doi.org/10.1146/annurev.biochem.69.1.183 - DOI - PubMed
-
- Munoz-Dorado J, Inouye S, Inouye M. A gene encoding a protein serine/threonine kinase is required for normal development of M. xanthus, a gram-negative bacterium. Cell 1991; 67:995-1006; PMID:1835671; http://dx.doi.org/10.1016/0092-8674(91)90372-6 - DOI - PubMed
-
- Galyov EE, Hakansson S, Forsberg A, Wolf-Watz H. A secreted protein kinase of Yersinia pseudotuberculosis is an indispensable virulence determinant. Nature 1993; 361:730-2; PMID:8441468; http://dx.doi.org/10.1038/361730a0 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
