Energy and molecules from photochemical/photocatalytic reactions. An overview
- PMID: 25603499
- PMCID: PMC6272209
- DOI: 10.3390/molecules20011527
Energy and molecules from photochemical/photocatalytic reactions. An overview
Abstract
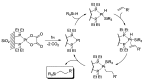
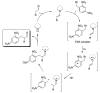
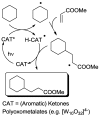
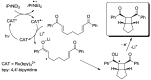
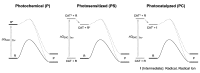
Photocatalytic reactions have been defined as those processes that require both a (not consumed) catalyst and light. A previous definition was whether such reactions brought a system towards or away from the (thermal) equilibrium. This consideration brings in the question whether a part of the photon energy is incorporated into the photochemical reaction products. Data are provided for representative organic reactions involving or not molecular catalysts and show that energy storage occurs only when a heavily strained structure is generated, and in that case only a minor part of photon energy is actually stored (ΔG up to 25 kcal·mol-1). The green role of photochemistry/photocatalysis is rather that of forming highly reactive intermediates under mild conditions.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
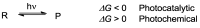


References
-
- Ciamician G. Actions chimiques de la lumière. Bull. Soc. Chim. Fr. 1908;3–4:i–xxvii.
-
- Albini A., Fagnoni M. Green chemistry and photochemistry were born at the same time. Green Chem. 2004;6:1–6.
-
- Albini A., Fagnoni M. 1908: Giacomo Ciamician and the concept of green chemistry. ChemSusChem. 2008;1:63–66. - PubMed
-
- Albini A., Dichiarante V. The belle époque of photochemistry. Photochem. Photobiol. Sci. 2009;8:248–254.
-
- Paternò E. Sintesi in chimica organica per mezzo della luce. Nota I. Introduzione. Gazz. Chim. Ital. 1914;44:31.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

