Spatiotemporal regulation of the anaphase-promoting complex in mitosis
- PMID: 25604195
- PMCID: PMC4386896
- DOI: 10.1038/nrm3934
Spatiotemporal regulation of the anaphase-promoting complex in mitosis
Abstract
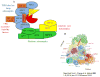
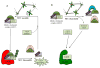
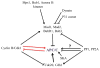
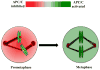
The appropriate timing of events that lead to chromosome segregation during mitosis and cytokinesis is essential to prevent aneuploidy, and defects in these processes can contribute to tumorigenesis. Key mitotic regulators are controlled through ubiquitylation and proteasome-mediated degradation. The APC/C (anaphase-promoting complex; also known as the cyclosome) is an E3 ubiquitin ligase that has a crucial function in the regulation of the mitotic cell cycle, particularly at the onset of anaphase and during mitotic exit. Co-activator proteins, inhibitor proteins, protein kinases and phosphatases interact with the APC/C to temporally and spatially control its activity and thus ensure accurate timing of mitotic events.
Figures


References
-
- Satyanarayana A, Kaldis P. Mammalian cell-cycle regulation: several Cdks, numerous cyclins and diverse compensatory mechanisms. Oncogene. 2009;28:2925–39. - PubMed
-
- Nakayama KI, Nakayama K. Regulation of the cell cycle by SCF-type ubiquitin ligases. Semin Cell Dev Biol. 2005;16:323–33. - PubMed
-
- Weissman AM. Themes and variations on ubiquitylation. Nat Rev Mol Cell Biol. 2001;2:169–78. - PubMed
-
- Nakayama KI, Nakayama K. Ubiquitin ligases: cell-cycle control and cancer. Nat Rev Cancer. 2006;6:369–81. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

