Characterization of human disease phenotypes associated with mutations in TREX1, RNASEH2A, RNASEH2B, RNASEH2C, SAMHD1, ADAR, and IFIH1
- PMID: 25604658
- PMCID: PMC4382202
- DOI: 10.1002/ajmg.a.36887
Characterization of human disease phenotypes associated with mutations in TREX1, RNASEH2A, RNASEH2B, RNASEH2C, SAMHD1, ADAR, and IFIH1
Abstract
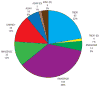
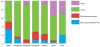
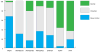
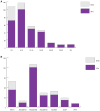
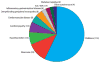
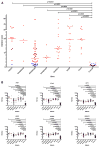
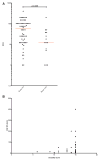
Aicardi-Goutières syndrome is an inflammatory disease occurring due to mutations in any of TREX1, RNASEH2A, RNASEH2B, RNASEH2C, SAMHD1, ADAR or IFIH1. We report on 374 patients from 299 families with mutations in these seven genes. Most patients conformed to one of two fairly stereotyped clinical profiles; either exhibiting an in utero disease-onset (74 patients; 22.8% of all patients where data were available), or a post-natal presentation, usually within the first year of life (223 patients; 68.6%), characterized by a sub-acute encephalopathy and a loss of previously acquired skills. Other clinically distinct phenotypes were also observed; particularly, bilateral striatal necrosis (13 patients; 3.6%) and non-syndromic spastic paraparesis (12 patients; 3.4%). We recorded 69 deaths (19.3% of patients with follow-up data). Of 285 patients for whom data were available, 210 (73.7%) were profoundly disabled, with no useful motor, speech and intellectual function. Chilblains, glaucoma, hypothyroidism, cardiomyopathy, intracerebral vasculitis, peripheral neuropathy, bowel inflammation and systemic lupus erythematosus were seen frequently enough to be confirmed as real associations with the Aicardi-Goutieres syndrome phenotype. We observed a robust relationship between mutations in all seven genes with increased type I interferon activity in cerebrospinal fluid and serum, and the increased expression of interferon-stimulated gene transcripts in peripheral blood. We recorded a positive correlation between the level of cerebrospinal fluid interferon activity assayed within one year of disease presentation and the degree of subsequent disability. Interferon-stimulated gene transcripts remained high in most patients, indicating an ongoing disease process. On the basis of substantial morbidity and mortality, our data highlight the urgent need to define coherent treatment strategies for the phenotypes associated with mutations in the Aicardi-Goutières syndrome-related genes. Our findings also make it clear that a window of therapeutic opportunity exists relevant to the majority of affected patients and indicate that the assessment of type I interferon activity might serve as a useful biomarker in future clinical trials.
Keywords: Aicardi-Goutières syndrome; bilateral striatal necrosis; interferon signature; spastic paraparesis; type I interferon.
© 2015 Wiley Periodicals, Inc.
Conflict of interest statement
Conflict of interest: none.
Figures
References
-
- Abe J, Nakamura K, Nishikomori R, Kato M, Mitsuiki N, Izawa K, Awaya T, Kawai T, Yasumi T, Toyoshima I, Hasegawa K, Ohshima Y, Hiragi T, Sasahara Y, Suzuki Y, Kikuchi M, Osaka H, Ohya T, Ninomiya S, Fujikawa S, Akasaka M, Iwata N, Kawakita A, Funatsuka M, Shintaku H, Ohara O, Ichinose H, Heike T. A nationwide survey of Aicardi-Goutieres syndrome patients identifies a strong association between dominant TREX1 mutations and chilblain lesions: Japanese cohort study. Rheumatology (Oxford) 2014;53:448–458. - PubMed
-
- Aicardi J, Goutieres F. A progressive familial encephalopathy in infancy with calcifications of the basal ganglia and chronic cerebrospinal fluid lymphocytosis. Ann Neurol. 1984;15:49–54. - PubMed
-
- Aicardi J, Goutieres F. Systemic lupus erythematosus or Aicardi-Goutieres syndrome. Neuropediatrics. 2000;31:113. - PubMed
-
- Clifford R, Louis T, Robbe P, Ackroyd S, Burns A, Timbs AT, Colopy Wright, Dreau G, Sigaux H, Judde F, Rotger JG, Telenti M, Lin A, Pasero YL, Maelfait P, Titsias J, Cohen M, Henderson DR, Ross SJ, Bentley MT, Hillmen D, Pettitt P, Rehwinkel A, Knight J, Taylor SJ, Crow JC, Benkirane YJ, Schuh M. SAMHD1 is mutated recurrently in chronic lymphocytic leukemia and is involved in response to DNA damage. Blood. 2014;123:1021–1031. - PMC - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

