Human Islet Morphology Revisited: Human and Rodent Islets Are Not So Different After All
- PMID: 25604813
- PMCID: PMC4530393
- DOI: 10.1369/0022155415570969
Human Islet Morphology Revisited: Human and Rodent Islets Are Not So Different After All
Abstract
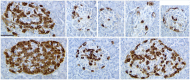
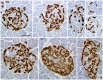
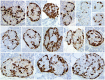
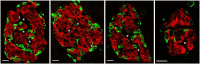
There has been great interest in understanding how human islets differ from rodent islets. Three major issues about human islet morphology have remained controversial over recent decades: 1) the proportion of the islet made up of β-cells; 2) whether islet cell types have a non-random mantle-core pattern, as seen in rodents, or are randomly scattered throughout the islet; 3) the relation of the different cell types to the blood vessels within the islet, which has implications for intraislet function. We re-examined these issues on immunostained sections of non-diabetic adult human pancreas. The composition of the islets can vary by the analysis method (number vs volume) and by the sampling of islets by size. The majority of adult human islets have clear, non-random clustering of β-cells and blood vessels that penetrate into the β-cell cores. We conclude that although there is far more variability in islet composition both within each human pancreas and among different human pancreas than in rodent pancreas, the islet architecture is not so different between the species. The intrapancreatic variability raises important questions about how islets evolve and function throughout life and how this might relate to the pathogenesis of diabetes.
Keywords: human islets; islet architecture; islet composition; mantle-core.
© The Author(s) 2015.
Conflict of interest statement
Figures
References
-
- Bonner-Weir S. (1988). Morphological evidence for pancreatic polarity of B-cell within the islets of Langerhans. Diabetes 37:616-621. - PubMed
-
- Bonner-Weir S, Orci L. (1982). New perspectives on the microvasculature of the islets of Langerhans in the rat. Diabetes 31:883-939. - PubMed
-
- Brissova M, Fowler MJ, Nicholson WE, Chu A, Hirshberg B, Harlan DM, Powers AC. (2005). Assessment of human pancreatic islet architecture and composition by laser scanning confocal microscopy. J Histochem Cytochem 53:1087-1097. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

