Angiogenin secretion from hepatoma cells activates hepatic stellate cells to amplify a self-sustained cycle promoting liver cancer
- PMID: 25604905
- PMCID: PMC4300499
- DOI: 10.1038/srep07916
Angiogenin secretion from hepatoma cells activates hepatic stellate cells to amplify a self-sustained cycle promoting liver cancer
Abstract
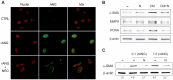
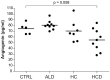
Hepatocellular carcinoma (HCC) frequently develops in a pro-inflammatory and pro-fibrogenic environment with hepatic stellate cells (HSCs) remodeling the extracellular matrix composition. Molecules secreted by liver tumors contributing to HSC activation and peritumoral stromal transformation remain to be fully identified. Here we show that conditioned medium from HCC cell lines, Hep3B and HepG2, induced primary mouse HSCs transdifferentiation, characterized by profibrotic properties and collagen modification, with similar results seen in the human HSC cell line LX2. Moreover, tumor growth was enhanced by coinjection of HepG2/LX2 cells in a xenograft murine model, supporting a HCC-HSC crosstalk in liver tumor progression. Protein microarray secretome analyses revealed angiogenin as the most robust and selective protein released by HCC compared to LX2 secreted molecules. In fact, recombinant angiogenin induced in vitro HSC activation requiring its nuclear translocation and rRNA transcriptional stimulation. Moreover, angiogenin antagonism by blocking antibodies or angiogenin inhibitor neomycin decreased in vitro HSC activation by conditioned media or recombinant angiogenin. Finally, neomycin administration reduced tumor growth of HepG2-LX2 cells coinjected in mice. In conclusion, angiogenin secretion by HCCs favors tumor development by inducing HSC activation and ECM remodeling. These findings indicate that targeting angiogenin signaling may be of potential relevance in HCC management.
Figures






References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

