Molecular chaperone GRP78 enhances aggresome delivery to autophagosomes to promote drug resistance in multiple myeloma
- PMID: 25605012
- PMCID: PMC4413640
- DOI: 10.18632/oncotarget.3075
Molecular chaperone GRP78 enhances aggresome delivery to autophagosomes to promote drug resistance in multiple myeloma
Abstract
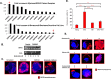
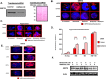
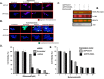
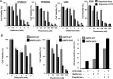
Despite the clinical benefit of the proteasome inhibitor bortezomib, multiple myeloma (MM) patients invariably relapse through poorly defined mechanisms. Myeloma cells inevitably develop chemoresistance that leads to disease relapse and patient-related deaths. Studies in tumor cell lines and biopsies obtained from patients refractory to therapy have revealed that myeloma cells adapt to stress by inducing expression of glucose-regulated protein 78 (GRP78), an endoplasmic reticulum (ER) chaperone with anti-apoptotic properties. Treatment of myeloma cells with bortezomib increased GRP78 levels and activated GRP78-dependent autophagy. Expression profiling indicated that GRP78-encoding HSPA5 was significantly upregulated in bortezomib-resistant cells. Co-treatment with the anti-diabetic agent metformin suppressed GRP78 and enhanced the anti-proliferative effect of bortezomib. Bortezomib treatment led to GRP78 co-localization with proteotoxic protein aggregates, known as aggresomes. Pharmacologic suppression, genetic ablation or mutational inactivation of GRP78 followed by bortezomib treatment led to the accumulation of aggresomes but impaired autophagy and enhanced anti-myeloma effect of bortezomib. GRP78 was co-immunoprecipitated with the KDEL receptor, an ER quality control regulator that binds proteins bearing the KDEL motif to mediate their retrieval from the Golgi complex back to the ER. Taken together, we demonstrate that inhibition of GRP78 functional activity disrupts autophagy and enhances the anti-myeloma effect of bortezomib.
Conflict of interest statement
The authors report no conflicts of interest pertaining to this manuscript.
Figures

References
-
- Palumbo A, Anderson K. Multiple myeloma. N Engl J Med. 2011;364:1046–1060. - PubMed
-
- Becker N. Epidemiology of multiple myeloma. Recent Results Cancer Res. 2011;183:25–35. - PubMed
-
- Reeder CB, Reece DE, Kukreti V, Mikhael JR, Chen C, Trudel S, Laumann K, Vohra H, Fonseca R, Bergsagel PL, Leis JF, Tiedemann R, Stewart AK. Long-term survival with cyclophosphamide, bortezomib and dexamethasone induction therapy in patients with newly diagnosed multiple myeloma. Br J Hematol. 2014;167(4):563–565. - PubMed
-
- http://seer.cancer.gov/statfacts/html/mulmy.html NCI surveillance, epidemiology and end results program.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

