Folate-mediated mitochondrial targeting with doxorubicin-polyrotaxane nanoparticles overcomes multidrug resistance
- PMID: 25605018
- PMCID: PMC4413620
- DOI: 10.18632/oncotarget.3090
Folate-mediated mitochondrial targeting with doxorubicin-polyrotaxane nanoparticles overcomes multidrug resistance
Abstract
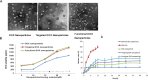
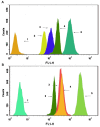
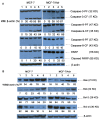
Resistance to treatment with anticancer drugs is a signiï¬cant obstacle and a fundamental cause of therapeutic failure in cancer therapy. Functional doxorubicin (DOX) nanoparticles for targeted delivery of the classical cytotoxic anticancer drug DOX to tumor cells, using folate-terminated polyrotaxanes along with dequalinium, have been developed and proven to overcome this resistance due to specific molecular features, including a size of approximately 101 nm, a zeta potential of 3.25 mV and drug-loading content of 18%. Compared with free DOX, DOX hydrochloride, DOX nanoparticles, and targeted DOX nanoparticles, the functional DOX nanoparticles exhibited the strongest anticancer efï¬cacy in vitro and in the drug-resistant MCF-7/Adr (DOX) xenograft tumor model. More specifically, the nanoparticles signiï¬cantly increased the intracellular uptake of DOX, selectively accumulating in mitochondria and the endoplasmic reticulum after treatment, with release of cytochrome C as a result. Furthermore, the caspase-9 and caspase-3 cascade was activated by the functional DOX nanoparticles through upregulation of the pro-apoptotic proteins Bax and Bid and suppression of the antiapoptotic protein Bcl-2, thereby enhancing apoptosis by acting on the mitochondrial signaling pathways. In conclusion, functional DOX nanoparticles may provide a strategy for increasing the solubility of DOX and overcoming multidrug-resistant cancers.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






References
-
- Ozben T. Mechanisms and strategies to overcome multiple drug resistance in cancer. FEBS letters. 2006;580(12):2903–2909. - PubMed
-
- Wong ST, Goodin S. Overcoming drug resistance in patients with metastatic breast cancer. Pharmacotherapy. 2009;29(8):954–965. - PubMed
-
- Green DR, Reed JC. Mitochondria and apoptosis. Science. 1998;281(5381):1309–1312. - PubMed
-
- Brenner C, Kroemer G. Apoptosis. Mitochondria--the death signal integrators. Science. 2000;289(5482):1150–1151. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

