MicroRNA 196B regulates FAS-mediated apoptosis in colorectal cancer cells
- PMID: 25605245
- PMCID: PMC4413621
- DOI: 10.18632/oncotarget.3066
MicroRNA 196B regulates FAS-mediated apoptosis in colorectal cancer cells
Abstract
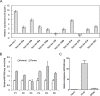
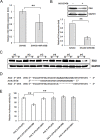
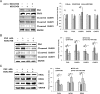
Using miRNA microarray analysis, we identified 31 miRNAs that were significantly up-regulated or down-regulated in colon cancer tissues. We chose MIR196B, which was specifically up-regulated in colon cancer, for further study. We identified 18 putative MIR196B target genes by comparing between the mRNAs down-regulated in MIR196B-overexpressed cells and the assumed MIR196B target genes predicted by public bioinformatics tools. The association between MIR196B and FAS was verified in this study. FAS expression was constitutively elevated in normal human colorectal tissues. However, its expression was often reduced in human colorectal cancer. The decrease in FAS expression could be responsible for the reduction of apoptosis in colorectal cancer cells. In colorectal cancer tissue, we showed that MIR196B up-regulation was mutually followed by down regulation of FAS expression. We also showed that MIR196B directly repressed FAS expression in colorectal cells. Furthermore, anti-MIR196B up-regulated FAS expression and increased apoptosis in colorectal cancer cell lines. Our results suggest that the up-regulation of MIR196B modulates apoptosis in colorectal cancer cells by partially repressing FAS expression and that anti-MIR196B could be a potential candidate as an anti-cancer drug in colorectal cancer therapy.
Conflict of interest statement
The authors have no competing interest to declare
Figures

References
-
- Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA: A Cancer Journal for Clinicians. 2005;55(2):74–108. - PubMed
-
- Swan J, Breen N, Coates RJ, Rimer BK, Lee NC. Progress in cancer screening practices in the United States: results from the 2000 National Health Interview Survey. Cancer. 2003;97:1528–1540. - PubMed
-
- Giovannucci E. Modifiable risk factors for colon cancer. Gastroenterology Clinics of North America. 2002;31(4):925–943. - PubMed
-
- Larsson SC, Orsini N, Wolk A. Diabetes mellitus and risk of colorectal cancer: a meta-analysis. Journal of the National Cancer Institute. 2005;97(22):1679–1687. - PubMed
-
- Itzkowitz SH, Yio X. Inflammation and cancer IV. Colorectal cancer in inflammatory bowel disease: the role of inflammation. American Journal of Physiology - Gastrointestinal and Liver Physiology. 2004;287(1):G7–17. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

