The use of murine-derived fundic organoids in studies of gastric physiology
- PMID: 25605613
- PMCID: PMC4405744
- DOI: 10.1113/jphysiol.2014.283028
The use of murine-derived fundic organoids in studies of gastric physiology
Abstract
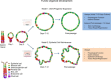
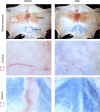
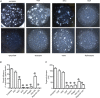
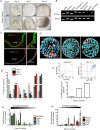
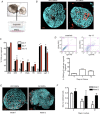
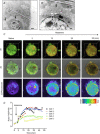
Key points: An in vitro approach to study gastric development is primary mouse-derived epithelium cultured as three-dimensional spheroids known as organoids. We have devised two unique gastric fundic-derived organoid cultures: model 1 for the expansion of gastric fundic stem cells, and model 2 for the maintenance of mature cell lineages. Organoids maintained in co-culture with immortalized stomach mesenchymal cells express robust numbers of surface pit, mucous neck, chief, endocrine and parietal cells. Histamine induced a significant decrease in intraluminal pH that was reversed by omeprazole in fundic organoids and indicated functional activity and regulation of parietal cells. Localized photodamage resulted in rapid cell exfoliation coincident with migration of neighbouring cells to the damaged area, sustaining epithelial continuity. We report the use of these models for studies of epithelial cell biology and cell damage and repair.
Abstract: Studies of gastric function and disease have been limited by the lack of extended primary cultures of the epithelium. An in vitro approach to study gastric development is primary mouse-derived antral epithelium cultured as three-dimensional spheroids known as organoids. There have been no reports on the use of organoids for gastric function. We have devised two unique gastric fundic-derived organoid cultures: model 1 for the expansion of gastric fundic stem cells, and model 2 for the maintenance of mature cell lineages. Both models were generated from single glands dissociated from whole fundic tissue and grown in basement membrane matrix (Matrigel) and organoid growth medium. Model 1 enriches for a stem cell-like niche via simple passage of the organoids. Maintained in Matrigel and growth medium, proliferating organoids expressed high levels of stem cell markers CD44 and Lgr5. Model 2 is a system of gastric organoids co-cultured with immortalized stomach mesenchymal cells (ISMCs). Organoids maintained in co-culture with ISMCs express robust numbers of surface pit, mucous neck, chief, endocrine and parietal cells. Histamine induced a significant decrease in intraluminal pH that was reversed by omeprazole in fundic organoids and indicated functional activity and regulation of parietal cells. Localized photodamage resulted in rapid cell exfoliation coincident with migration of neighbouring cells to the damaged area, sustaining epithelial continuity. Thus, we report the use of these models for studies of epithelial cell biology and cell damage and repair.
© 2015 The Authors. The Journal of Physiology © 2015 The Physiological Society.
Figures




References
-
- Aruffo A, Stamenkovic I, Melnick M, Underhill CB. Seed B. CD44 is the principal cell surface receptor for hyaluronate. Cell. 1990;61:1303–1313. - PubMed
-
- Barker N, Huch M, Kujala P, van de Wetering M, Snippert H, van Es JH, Sato T, Stange DE, Begthel H, van den Born M, Danenberg E, van den Brink S, Korving J, Abo A, Peters PJ, Wright N, Poulsom R. Clevers H. Lgr5(+ve) stem cells drive self-renewal in the stomach and build long-lived gastric units in vitro. Cell Stem Cell. 2010;6:25–36. - PubMed
-
- Bell SM, Schreiner CM, Wert SE, Mucenski ML, Scott WJ. Whitsett JA. R spondin 2 is required for normal laryngeal–tracheal, lung and limb morphogenesis. Development. 2008;135:1049–1058. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

