Microfluidic model of ductal carcinoma in situ with 3D, organotypic structure
- PMID: 25605670
- PMCID: PMC4305264
- DOI: 10.1186/s12885-015-1007-5
Microfluidic model of ductal carcinoma in situ with 3D, organotypic structure
Abstract
Background: Ductal carcinoma in situ (DCIS) is a non-invasive form of breast cancer that is thought to be a precursor to most invasive and metastatic breast cancers. Understanding the mechanisms regulating the invasive transition of DCIS is critical in order to better understand how some types of DCIS become invasive. While significant insights have been gained using traditional in vivo and in vitro models, existing models do not adequately recapitulate key structure and functions of human DCIS well. In addition, existing models are time-consuming and costly, limiting their use in routine screens. Here, we present a microscale DCIS model that recapitulates key structures and functions of human DCIS, while enhancing the throughput capability of the system to simultaneously screen numerous molecules and drugs.
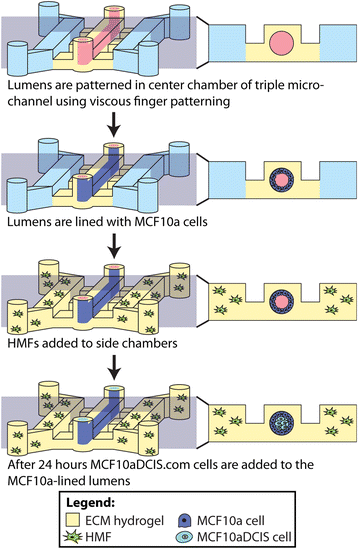
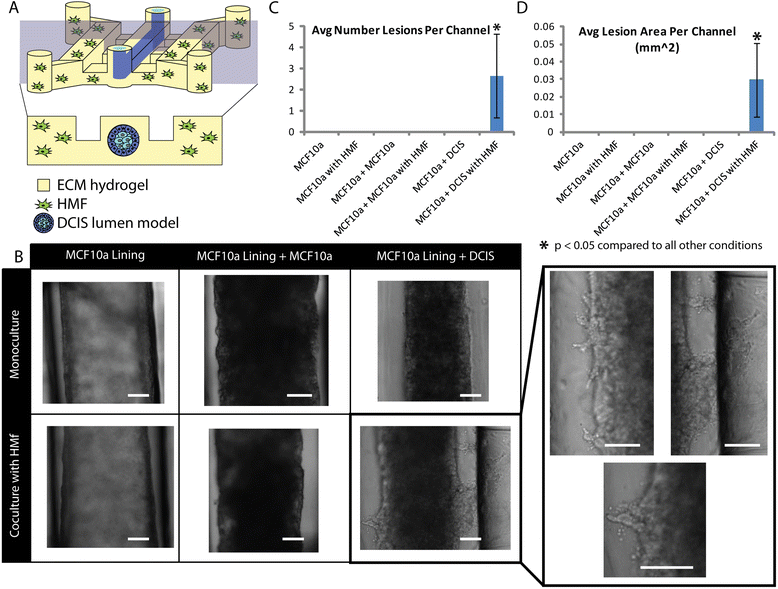
Methods: Our microscale DCIS model is prepared in two steps. First, viscous finger patterning is used to generate mammary epithelial cell-lined lumens through extracellular matrix hydrogels. Next, DCIS cells are added to fill the mammary ducts to create a DCIS-like structure. For coculture experiments, human mammary fibroblasts (HMF) are added to the two side channels connected to the center channel containing DCIS. To validate the invasive transition of the DCIS model, the invasion of cancer cells and the loss of cell-cell junctions are then examined. A student t-test is conducted for statistical analysis.
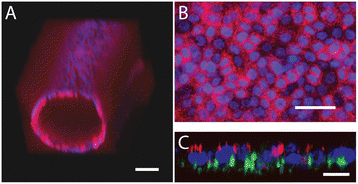
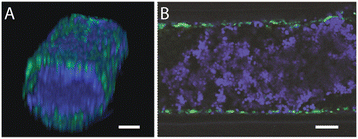
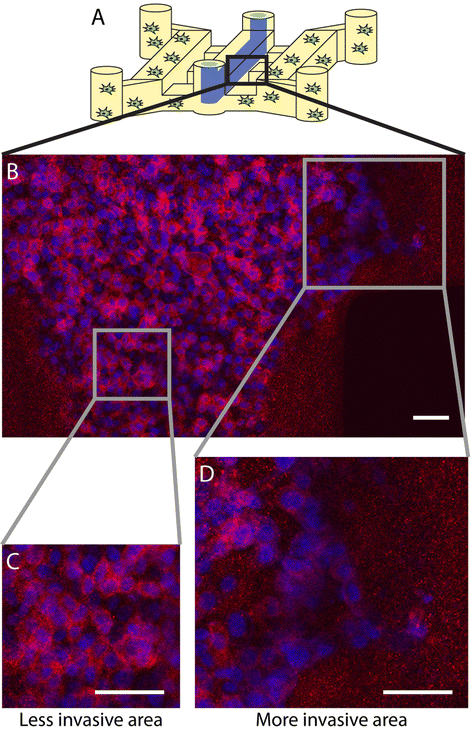
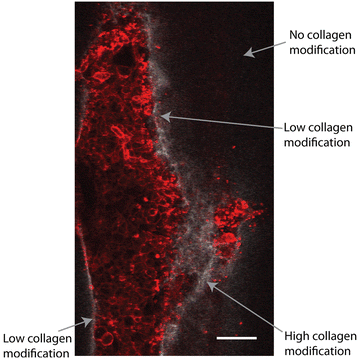
Results: We demonstrate that our DCIS model faithfully recapitulates key structures and functions of human mammary DCIS and can be employed to study the mechanisms involved in the invasive progression of DCIS. First, the formation of cell-cell junctions and cell polarity in the normal mammary duct, and the structure of the DCIS model are characterized. Second, coculture with HMF is shown to induce the invasion of DCIS. Third, multiple endpoint analyses are demonstrated to validate the invasion.
Conclusions: We have developed and characterized a novel in vitro model of normal and DCIS-inflicted mammary ducts with 3D lumen structures. These models will enable researchers to investigate the role of microenvironmental factors on the invasion of DCIS in more in vivo-like conditions.
Figures
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

