Apparent role for Borrelia burgdorferi LuxS during mammalian infection
- PMID: 25605770
- PMCID: PMC4363420
- DOI: 10.1128/IAI.00032-15
Apparent role for Borrelia burgdorferi LuxS during mammalian infection
Abstract
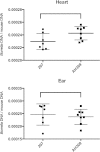
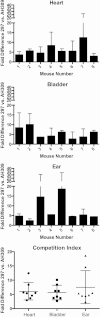
The Lyme disease spirochete, Borrelia burgdorferi, controls protein expression patterns during its tick-mammal infection cycle. Earlier studies demonstrated that B. burgdorferi synthesizes 4,5-dihydroxy-2,3-pentanedione (autoinducer-2 [AI-2]) and responds to AI-2 by measurably changing production of several infection-associated proteins. luxS mutants, which are unable to produce AI-2, exhibit altered production of several proteins. B. burgdorferi cannot utilize the other product of LuxS, homocysteine, indicating that phenotypes of luxS mutants are not due to the absence of that molecule. Although a previous study found that a luxS mutant was capable of infecting mice, a critical caveat to those results is that bacterial loads were not quantified. To more precisely determine whether LuxS serves a role in mammalian infection, mice were simultaneously inoculated with congenic wild-type and luxS strains, and bacterial numbers were assessed using quantitative PCR. The wild-type bacteria substantially outcompeted the mutants, suggesting that LuxS performs a significant function during mammalian infection. These data also provide further evidence that nonquantitative infection studies do not necessarily provide conclusive results and that regulatory factors may not make all-or-none, black-or-white contributions to infectivity.
Copyright © 2015, American Society for Microbiology. All Rights Reserved.
Figures
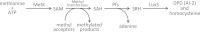

Similar articles
-
Functionality of Borrelia burgdorferi LuxS: the Lyme disease spirochete produces and responds to the pheromone autoinducer-2 and lacks a complete activated-methyl cycle.Int J Med Microbiol. 2006 May;296 Suppl 40:92-102. doi: 10.1016/j.ijmm.2005.12.011. Epub 2006 Mar 10. Int J Med Microbiol. 2006. PMID: 16530477
-
Expression of a luxS gene is not required for Borrelia burgdorferi infection of mice via needle inoculation.Infect Immun. 2003 May;71(5):2892-6. doi: 10.1128/IAI.71.5.2892-2896.2003. Infect Immun. 2003. PMID: 12704164 Free PMC article.
-
Synthesis of autoinducer 2 by the lyme disease spirochete, Borrelia burgdorferi.J Bacteriol. 2005 May;187(9):3079-87. doi: 10.1128/JB.187.9.3079-3087.2005. J Bacteriol. 2005. PMID: 15838035 Free PMC article.
-
The luxS gene is not required for Borrelia burgdorferi tick colonization, transmission to a mammalian host, or induction of disease.Infect Immun. 2004 Aug;72(8):4864-7. doi: 10.1128/IAI.72.8.4864-4867.2004. Infect Immun. 2004. PMID: 15271949 Free PMC article.
-
Quorum sensing by the Lyme disease spirochete.Microbes Infect. 2003 Sep;5(11):991-7. doi: 10.1016/s1286-4579(03)00184-9. Microbes Infect. 2003. PMID: 12941391 Review.
Cited by
-
Interference With Quorum-Sensing Signal Biosynthesis as a Promising Therapeutic Strategy Against Multidrug-Resistant Pathogens.Front Cell Infect Microbiol. 2019 Feb 5;8:444. doi: 10.3389/fcimb.2018.00444. eCollection 2018. Front Cell Infect Microbiol. 2019. PMID: 30805311 Free PMC article. Review.
-
Lyme Disease Frontiers: Reconciling Borrelia Biology and Clinical Conundrums.Pathogens. 2019 Dec 16;8(4):299. doi: 10.3390/pathogens8040299. Pathogens. 2019. PMID: 31888245 Free PMC article. Review.
-
Borreliella burgdorferi Antimicrobial-Tolerant Persistence in Lyme Disease and Posttreatment Lyme Disease Syndromes.mBio. 2022 Jun 28;13(3):e0344021. doi: 10.1128/mbio.03440-21. Epub 2022 Apr 25. mBio. 2022. PMID: 35467428 Free PMC article. Review.
-
Pathogenicity and virulence of Borrelia burgdorferi.Virulence. 2023 Dec;14(1):2265015. doi: 10.1080/21505594.2023.2265015. Epub 2023 Oct 9. Virulence. 2023. PMID: 37814488 Free PMC article. Review.
-
Lyme Disease Pathogenesis.Curr Issues Mol Biol. 2021;42:473-518. doi: 10.21775/cimb.042.473. Epub 2020 Dec 23. Curr Issues Mol Biol. 2021. PMID: 33353871 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

