Molecular mechanisms in DM1 - a focus on foci
- PMID: 25605794
- PMCID: PMC4344492
- DOI: 10.1093/nar/gkv029
Molecular mechanisms in DM1 - a focus on foci
Abstract
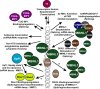
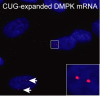
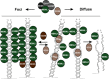
Myotonic dystrophy type 1 is caused by abnormal expansion of a CTG-trinucleotide repeat in the gene encoding Dystrophia Myotonica Protein Kinase (DMPK), which in turn leads to global deregulation of gene expression in affected individuals. The transcribed mRNA contains a massive CUG-expansion in the 3' untranslated region (3'UTR) facilitating nucleation of several regulatory RNA-binding proteins, which are thus unable to perform their normal cellular function. These CUG-expanded mRNA-protein aggregates form distinct, primarily nuclear foci. In differentiated muscle cells, most of the CUG-expanded RNA remains in the nuclear compartment, while in dividing cells such as fibroblasts a considerable fraction of the mutant RNA reaches the cytoplasm, consistent with findings that both nuclear and cytoplasmic events are mis-regulated in DM1. Recent evidence suggests that the nuclear aggregates, or ribonuclear foci, are more dynamic than previously anticipated and regulated by several proteins, including RNA helicases. In this review, we focus on the homeostasis of DMPK mRNA foci and discuss how their dynamic regulation may affect disease-causing mechanisms in DM1.
© The Author(s) 2015. Published by Oxford University Press on behalf of Nucleic Acids Research.
Figures
References
-
- Turner C., Hilton-Jones D. Myotonic dystrophy: diagnosis, management and new therapies. Curr. Opin. Neurol. 2014;27:599–606. - PubMed
-
- Udd B., Krahe R. The myotonic dystrophies: molecular, clinical, and therapeutic challenges. Lancet Neurol. 2012;11:891–905. - PubMed
-
- Meola G., Jones K., Wei C., Timchenko L.T. Dysfunction of protein homeostasis in myotonic dystrophies. Histol. Histopathol. 2013;28:1089–1098. - PubMed
-
- Brook J.D., McCurrach M.E., Harley H.G., Buckler A.J., Church D., Aburatani H., Hunter K., Stanton V.P., Thirion J.P., Hudson T., et al. Molecular basis of myotonic dystrophy: expansion of a trinucleotide (CTG) repeat at the 3′ end of a transcript encoding a protein kinase family member. Cell. 1992;68:799–808. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

