Dipeptidyl peptidase-4 inhibition ameliorates Western diet-induced hepatic steatosis and insulin resistance through hepatic lipid remodeling and modulation of hepatic mitochondrial function
- PMID: 25605806
- PMCID: PMC4439570
- DOI: 10.2337/db14-0804
Dipeptidyl peptidase-4 inhibition ameliorates Western diet-induced hepatic steatosis and insulin resistance through hepatic lipid remodeling and modulation of hepatic mitochondrial function
Abstract
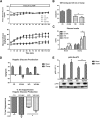
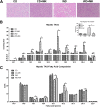
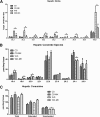
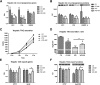
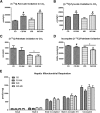
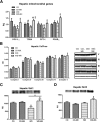
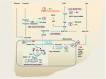
Novel therapies are needed for treating the increasing prevalence of hepatic steatosis in Western populations. In this regard, dipeptidyl peptidase-4 (DPP-4) inhibitors have recently been reported to attenuate the development of hepatic steatosis, but the potential mechanisms remain poorly defined. In the current study, 4-week-old C57Bl/6 mice were fed a high-fat/high-fructose Western diet (WD) or a WD containing the DPP-4 inhibitor, MK0626, for 16 weeks. The DPP-4 inhibitor prevented WD-induced hepatic steatosis and reduced hepatic insulin resistance by enhancing insulin suppression of hepatic glucose output. WD-induced accumulation of hepatic triacylglycerol (TAG) and diacylglycerol (DAG) content was significantly attenuated with DPP-4 inhibitor treatment. In addition, MK0626 significantly reduced mitochondrial incomplete palmitate oxidation and increased indices of pyruvate dehydrogenase activity, TCA cycle flux, and hepatic TAG secretion. Furthermore, DPP-4 inhibition rescued WD-induced decreases in hepatic PGC-1α and CPT-1 mRNA expression and hepatic Sirt1 protein content. Moreover, plasma uric acid levels in mice fed the WD were decreased after MK0626 treatment. These studies suggest that DPP-4 inhibition ameliorates hepatic steatosis and insulin resistance by suppressing hepatic TAG and DAG accumulation through enhanced mitochondrial carbohydrate utilization and hepatic TAG secretion/export with a concomitant reduction of uric acid production.
© 2015 by the American Diabetes Association. Readers may use this article as long as the work is properly cited, the use is educational and not for profit, and the work is not altered.
Figures
Similar articles
-
Prevention and treatment effect of evogliptin on hepatic steatosis in high-fat-fed animal models.Arch Pharm Res. 2017 Feb;40(2):268-281. doi: 10.1007/s12272-016-0864-z. Epub 2016 Nov 24. Arch Pharm Res. 2017. PMID: 27885461
-
Gemigliptin ameliorates Western-diet-induced metabolic syndrome in mice.Can J Physiol Pharmacol. 2017 Feb;95(2):129-139. doi: 10.1139/cjpp-2016-0026. Epub 2016 Sep 4. Can J Physiol Pharmacol. 2017. PMID: 27918207
-
A dipeptidyl peptidase-IV inhibitor improves hepatic steatosis and insulin resistance by AMPK-dependent and JNK-dependent inhibition of LECT2 expression.Biochem Pharmacol. 2015 Nov 1;98(1):157-66. doi: 10.1016/j.bcp.2015.08.098. Epub 2015 Aug 20. Biochem Pharmacol. 2015. PMID: 26297911
-
Dipeptidyl peptidase-4: a key player in chronic liver disease.World J Gastroenterol. 2013 Apr 21;19(15):2298-306. doi: 10.3748/wjg.v19.i15.2298. World J Gastroenterol. 2013. PMID: 23613622 Free PMC article. Review.
-
Hepatic triacylglycerol accumulation and insulin resistance.J Lipid Res. 2009 Apr;50 Suppl(Suppl):S74-9. doi: 10.1194/jlr.R800053-JLR200. Epub 2008 Nov 6. J Lipid Res. 2009. PMID: 18997164 Free PMC article. Review.
Cited by
-
Prediabetes blunts DPP4 genetic control of postprandial glycaemia and insulin secretion.Diabetologia. 2022 May;65(5):861-871. doi: 10.1007/s00125-021-05638-6. Epub 2022 Feb 22. Diabetologia. 2022. PMID: 35190847 Free PMC article.
-
Elevated hepatic DPP4 activity promotes insulin resistance and non-alcoholic fatty liver disease.Mol Metab. 2017 Oct;6(10):1254-1263. doi: 10.1016/j.molmet.2017.07.016. Epub 2017 Aug 4. Mol Metab. 2017. PMID: 29031724 Free PMC article.
-
Efficacy and safety of incretin-based therapies in patients with nonalcoholic fatty liver disease: A protocol for a systematic review and meta-analysis.Medicine (Baltimore). 2020 Jul 2;99(27):e20695. doi: 10.1097/MD.0000000000020695. Medicine (Baltimore). 2020. PMID: 32629641 Free PMC article.
-
Pharmacology of dipeptidyl peptidase-4 inhibitors and its use in the management of metabolic syndrome: a comprehensive review on drug repositioning.Daru. 2019 Jun;27(1):341-360. doi: 10.1007/s40199-019-00238-7. Epub 2019 Jan 23. Daru. 2019. PMID: 30674032 Free PMC article. Review.
-
Dipeptidyl dipeptidase-4 inhibitor recovered ischemia through an increase in vasculogenic endothelial progenitor cells and regeneration-associated cells in diet-induced obese mice.PLoS One. 2019 Mar 19;14(3):e0205477. doi: 10.1371/journal.pone.0205477. eCollection 2019. PLoS One. 2019. PMID: 30889182 Free PMC article.
References
-
- Flegal KM, Carroll MD, Ogden CL, Curtin LR. Prevalence and trends in obesity among US adults, 1999-2008. JAMA 2010;303:235–241 - PubMed
-
- Barrera F, George J. The role of diet and nutritional intervention for the management of patients with NAFLD. Clin Liver Dis 2014;18:91–112 - PubMed
-
- Vos MB, Lavine JE. Dietary fructose in nonalcoholic fatty liver disease. Hepatology 2013;57:2525–2531 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 DA021299/DA/NIDA NIH HHS/United States
- R21 HL111906/HL/NHLBI NIH HHS/United States
- R01 HL073101/HL/NHLBI NIH HHS/United States
- HL-074214/HL/NHLBI NIH HHS/United States
- HL-73101-07/HL/NHLBI NIH HHS/United States
- R01 HL074214/HL/NHLBI NIH HHS/United States
- HL-111906/HL/NHLBI NIH HHS/United States
- I01 BX001981/BX/BLRD VA/United States
- R01 DK088940/DK/NIDDK NIH HHS/United States
- HL-107910-03/HL/NHLBI NIH HHS/United States
- R01 HL107910/HL/NHLBI NIH HHS/United States
- DK-088940/DK/NIDDK NIH HHS/United States
- U24 DK059637/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

