Biomechanical basis of wing and haltere coordination in flies
- PMID: 25605915
- PMCID: PMC4321282
- DOI: 10.1073/pnas.1412279112
Biomechanical basis of wing and haltere coordination in flies
Abstract
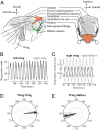
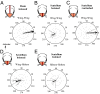
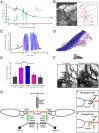
The spectacular success and diversification of insects rests critically on two major evolutionary adaptations. First, the evolution of flight, which enhanced the ability of insects to colonize novel ecological habitats, evade predators, or hunt prey; and second, the miniaturization of their body size, which profoundly influenced all aspects of their biology from development to behavior. However, miniaturization imposes steep demands on the flight system because smaller insects must flap their wings at higher frequencies to generate sufficient aerodynamic forces to stay aloft; it also poses challenges to the sensorimotor system because precise control of wing kinematics and body trajectories requires fast sensory feedback. These tradeoffs are best studied in Dipteran flies in which rapid mechanosensory feedback to wing motor system is provided by halteres, reduced hind wings that evolved into gyroscopic sensors. Halteres oscillate at the same frequency as and precisely antiphase to the wings; they detect body rotations during flight, thus providing feedback that is essential for controlling wing motion during aerial maneuvers. Although tight phase synchrony between halteres and wings is essential for providing proper timing cues, the mechanisms underlying this coordination are not well understood. Here, we identify specific mechanical linkages within the thorax that passively mediate both wing-wing and wing-haltere phase synchronization. We demonstrate that the wing hinge must possess a clutch system that enables flies to independently engage or disengage each wing from the mechanically linked thorax. In concert with a previously described gearbox located within the wing hinge, the clutch system enables independent control of each wing. These biomechanical features are essential for flight control in flies.
Keywords: halteres; insect thorax; insect wings; wing clutch; wing hinge.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Polilov AA. The smallest insects evolve anucleate neurons. Arthropod Struct Dev. 2012;41(1):29–34. - PubMed
-
- Dudley R. The Biomechanics of Insect Flight. Princeton Univ Press; Princeton, NJ: 2000.
-
- Josephson RK, Malamud JG, Stokes DR. Asynchronous muscle: A primer. J Exp Biol. 2000;203(18):2713–2722. - PubMed
-
- Dickinson MH, Tu MS. The function of dipteran flight muscle. Comp Biochem Physiol A. 1997;116(3):223–238.
-
- Bastian J, Esch H. Nervous control of indirect flight muscles of honey bee. Z Vgl Physiol. 1970;67(3):307.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

