MITF drives endolysosomal biogenesis and potentiates Wnt signaling in melanoma cells
- PMID: 25605940
- PMCID: PMC4321275
- DOI: 10.1073/pnas.1424576112
MITF drives endolysosomal biogenesis and potentiates Wnt signaling in melanoma cells
Abstract
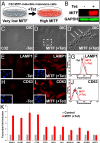
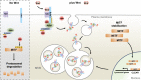
Canonical Wnt signaling plays an important role in development and disease, regulating transcription of target genes and stabilizing many proteins phosphorylated by glycogen synthase kinase 3 (GSK3). We observed that the MiT family of transcription factors, which includes the melanoma oncogene MITF (micropthalmia-associated transcription factor) and the lysosomal master regulator TFEB, had the highest phylogenetic conservation of three consecutive putative GSK3 phosphorylation sites in animal proteomes. This finding prompted us to examine the relationship between MITF, endolysosomal biogenesis, and Wnt signaling. Here we report that MITF expression levels correlated with the expression of a large subset of lysosomal genes in melanoma cell lines. MITF expression in the tetracycline-inducible C32 melanoma model caused a marked increase in vesicular structures, and increased expression of late endosomal proteins, such as Rab7, LAMP1, and CD63. These late endosomes were not functional lysosomes as they were less active in proteolysis, yet were able to concentrate Axin1, phospho-LRP6, phospho-β-catenin, and GSK3 in the presence of Wnt ligands. This relocalization significantly enhanced Wnt signaling by increasing the number of multivesicular bodies into which the Wnt signalosome/destruction complex becomes localized upon Wnt signaling. We also show that the MITF protein was stabilized by Wnt signaling, through the novel C-terminal GSK3 phosphorylations identified here. MITF stabilization caused an increase in multivesicular body biosynthesis, which in turn increased Wnt signaling, generating a positive-feedback loop that may function during the proliferative stages of melanoma. The results underscore the importance of misregulated endolysosomal biogenesis in Wnt signaling and cancer.
Keywords: MITF; Wnt-STOP; lysosome; melanoma; multivesicular body.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




Comment in
-
MITF regulation - more hints from Wnt.Pigment Cell Melanoma Res. 2015 Jul;28(4):372-3. doi: 10.1111/pcmr.12366. Epub 2015 Apr 13. Pigment Cell Melanoma Res. 2015. PMID: 25786516 No abstract available.
References
-
- Angers S, Moon RT. Proximal events in Wnt signal transduction. Nat Rev Mol Cell Biol. 2009;10(7):468–477. - PubMed
-
- Clevers H, Nusse R. Wnt/β-catenin signaling and disease. Cell. 2012;149(6):1192–1205. - PubMed
-
- Peifer M, Sweeton D, Casey M, Wieschaus E. Wingless signal and Zeste-white 3 kinase trigger opposing changes in the intracellular distribution of Armadillo. Development. 1994;120(2):369–380. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

