Neuroendocrine differentiation of prostate cancer: a review
- PMID: 25606573
- PMCID: PMC4297323
Neuroendocrine differentiation of prostate cancer: a review
Abstract
Neuroendocrine cells are one of the epithelial populations in the prostate. Neuroendocrine differentiation (NED) has been observed in prostate cancer. In addition to small cell neuroendocrine carcinomas and carcinoid tumors of the prostate, prostatic adenocarcinomas may have NED. The incidence and clinical relevance of NED in prostatic adenocarcinoma is not clearly understood because of conflicting results in the reported studies, and evaluation of NED is not routinely performed in clinical practice. This review is an overall synthesis with an aim to develop a more comprehensive understanding and practical approach towards the current knowledge of neuroendocrine differentiation. In this review we are stratifying these lesions into separate subtypes based on histologic parameters such as tumor morphology, neuroendocrine cell density and distribution and clinical parameters. We also want to identify current controversies and confusing issues not totally resolved in this topic for further investigations. Eventually a clearer understanding of this phenomenon and appropriate handling NED in prostate cancer will benefit clinical practice.
Keywords: Neuroendocrine differentiation; prostate.
Figures



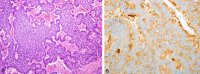


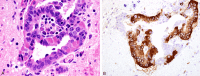
References
-
- Gretzer MB, Partin AW. PSA markers in prostate cancer detection. Urol Clin North Am. 2003;30:677–686. - PubMed
-
- Marchal C, Redondo M, Padilla M, Caballero J, Rodrigo I, Garcia J, Quian J, Boswick DG. Expression of prostate specific membrane antigen (PSMA) in prostatic adenocarcinoma and prostatic intraepithelial neoplasia. Histol Histopathol. 2004;19:715–718. - PubMed
-
- van Dieijen-Visser MP, Delaere KP, Gijzen AH, Brombacher PJ. A comparative study on the diagnostic value of prostatic acid phosphatase (PAP) and prostatic specific antigen (PSA) in patients with carcinoma of the prostate gland. Clin Chim Acta. 1988;174:131–140. - PubMed
-
- Aslan G, Irer B, Tuna B, Yorukoglu K, Saatcioglu F, Celebi I. Analysis of NKX3.1 expression in prostate cancer tissues and correlation with clinicopathologic features. Pathol Res Pract. 2006;202:93–98. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
