Platelet bioreactor-on-a-chip
- PMID: 25606631
- PMCID: PMC4168343
- DOI: 10.1182/blood-2014-05-574913
Platelet bioreactor-on-a-chip
Abstract
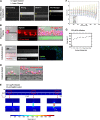
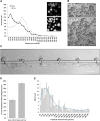
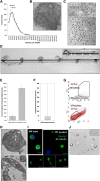
Platelet transfusions total >2.17 million apheresis-equivalent units per year in the United States and are derived entirely from human donors, despite clinically significant immunogenicity, associated risk of sepsis, and inventory shortages due to high demand and 5-day shelf life. To take advantage of known physiological drivers of thrombopoiesis, we have developed a microfluidic human platelet bioreactor that recapitulates bone marrow stiffness, extracellular matrix composition,micro-channel size, hemodynamic vascular shear stress, and endothelial cell contacts, and it supports high-resolution live-cell microscopy and quantification of platelet production. Physiological shear stresses triggered proplatelet initiation, reproduced ex vivo bone marrow proplatelet production, and generated functional platelets. Modeling human bone marrow composition and hemodynamics in vitro obviates risks associated with platelet procurement and storage to help meet growing transfusion needs.
Figures



Comment in
-
In vitro platelets in sight.Blood. 2014 Sep 18;124(12):1849-50. doi: 10.1182/blood-2014-08-592493. Blood. 2014. PMID: 25606626 No abstract available.
References
-
- Linden MD, Jackson DE. Platelets: pleiotropic roles in atherogenesis and atherothrombosis. Int J Biochem Cell Biol. 2010;42(11):1762–1766. - PubMed
-
- Semple JW, Italiano JE, Jr, Freedman J. Platelets and the immune continuum. Nat Rev Immunol. 2011;11(4):264–274. - PubMed
-
- Sullivan MT, Cotten R, Read EJ, Wallace EL. Blood collection and transfusion in the United States in 2001. Transfusion. 2007;47(3):385–394. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

