Molecular biophysics of Orai store-operated Ca2+ channels
- PMID: 25606672
- PMCID: PMC4302196
- DOI: 10.1016/j.bpj.2014.11.3473
Molecular biophysics of Orai store-operated Ca2+ channels
Abstract
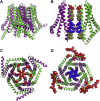
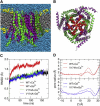
Upon endoplasmic reticulum Ca(2+) store depletion, Orai channels in the plasma membrane are activated directly by endoplasmic reticulum-resident STIM proteins to generate the Ca(2+)-selective, Ca(2+) release-activated Ca(2+) (CRAC) current. After the molecular identification of Orai, a plethora of functional and biochemical studies sought to compare Orai homologs, determine their stoichiometry, identify structural domains responsible for the biophysical fingerprint of the CRAC current, identify the physiological functions, and investigate Orai homologs as potential therapeutic targets. Subsequently, the solved crystal structure of Drosophila Orai (dOrai) substantiated many findings from structure-function studies, but also revealed an unexpected hexameric structure. In this review, we explore Orai channels as elucidated by functional and biochemical studies, analyze the dOrai crystal structure and its implications for Orai channel function, and present newly available information from molecular dynamics simulations that shed light on Orai channel gating and permeation.
Copyright © 2015 Biophysical Society. Published by Elsevier Inc. All rights reserved.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

