Molecular model for the solubilization of membranes into nanodisks by styrene maleic Acid copolymers
- PMID: 25606677
- PMCID: PMC4302193
- DOI: 10.1016/j.bpj.2014.11.3464
Molecular model for the solubilization of membranes into nanodisks by styrene maleic Acid copolymers
Abstract
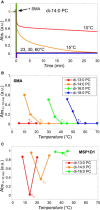
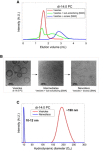
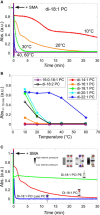
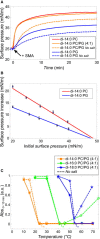
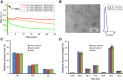
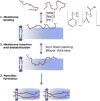
A recent discovery in membrane research is the ability of styrene-maleic acid (SMA) copolymers to solubilize membranes in the form of nanodisks allowing extraction and purification of membrane proteins from their native environment in a single detergent-free step. This has important implications for membrane research because it allows isolation as well as characterization of proteins and lipids in a near-native environment. Here, we aimed to unravel the molecular mode of action of SMA copolymers by performing systematic studies using model membranes of varying compositions and employing complementary biophysical approaches. We found that the SMA copolymer is a highly efficient membrane-solubilizing agent and that lipid bilayer properties such as fluidity, thickness, lateral pressure profile, and charge density all play distinct roles in the kinetics of solubilization. More specifically, relatively thin membranes, decreased lateral chain pressure, low charge density at the membrane surface, and increased salt concentration promote the speed and yield of vesicle solubilization. Experiments using a native membrane lipid extract showed that the SMA copolymer does not discriminate between different lipids and thus retains the native lipid composition in the solubilized particles. A model is proposed for the mode of action of SMA copolymers in which membrane solubilization is mainly driven by the hydrophobic effect and is further favored by physical properties of the polymer such as its relatively small cross-sectional area and rigid pendant groups. These results may be helpful for development of novel applications for this new type of solubilizing agent, and for optimization of the SMA technology for solubilization of the wide variety of cell membranes found in nature.
Copyright © 2015 Biophysical Society. Published by Elsevier Inc. All rights reserved.
Figures
References
-
- Knowles T.J., Finka R., Overduin M. Membrane proteins solubilized intact in lipid containing nanoparticles bounded by styrene maleic acid copolymer. J. Am. Chem. Soc. 2009;131:7484–7485. - PubMed
-
- Jamshad M., Lin Y.-P., Dafforn T.R. Surfactant-free purification of membrane proteins with intact native membrane environment. Biochem. Soc. Trans. 2011;39:813–818. - PubMed
-
- Orwick M.C., Judge P.J., Watts A. Detergent-free formation and physicochemical characterization of nanosized lipid-polymer complexes: Lipodisq. Angew. Chem. Int. Ed. Engl. 2012;51:4653–4657. - PubMed
-
- Orwick-Rydmark M., Lovett J.E., Watts A. Detergent-free incorporation of a seven-transmembrane receptor protein into nanosized bilayer Lipodisq particles for functional and biophysical studies. Nano Lett. 2012;12:4687–4692. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

