DNA charge transport within the cell
- PMID: 25606780
- PMCID: PMC4587570
- DOI: 10.1021/bi501520w
DNA charge transport within the cell
Abstract
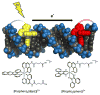
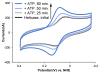
The unique characteristics of DNA charge transport (CT) have prompted an examination of roles for this chemistry within a biological context. Not only can DNA CT facilitate long-range oxidative damage of DNA, but redox-active proteins can couple to the DNA base stack and participate in long-range redox reactions using DNA CT. DNA transcription factors with redox-active moieties such as SoxR and p53 can use DNA CT as a form of redox sensing. DNA CT chemistry also provides a means to monitor the integrity of the DNA, given the sensitivity of DNA CT to perturbations in base stacking as arise with mismatches and lesions. Enzymes that utilize this chemistry include an interesting and ever-growing class of DNA-processing enzymes involved in DNA repair, replication, and transcription that have been found to contain 4Fe-4S clusters. DNA repair enzymes containing 4Fe-4S clusters, that include endonuclease III (EndoIII), MutY, and DinG from bacteria, as well as XPD from archaea, have been shown to be redox-active when bound to DNA, share a DNA-bound redox potential, and can be reduced and oxidized at long-range via DNA CT. Interactions between DNA and these proteins in solution, in addition to genetics experiments within Escherichia coli, suggest that DNA-mediated CT can be used as a means of cooperative signaling among DNA repair proteins that contain 4Fe-4S clusters as a first step in finding DNA damage, even within cells. On the basis of these data, we can consider also how DNA-mediated CT may be used as a means of signaling to coordinate DNA processing across the genome.
Figures


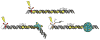
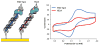



References
-
- Eley DD, Spivey DI. Semiconductivity of organic substances. Part 9. Nucleic acid in the dry state. Trans Faraday Soc. 1962;58:411–415.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

