Isoproterenol acts as a biased agonist of the alpha-1A-adrenoceptor that selectively activates the MAPK/ERK pathway
- PMID: 25606852
- PMCID: PMC4301629
- DOI: 10.1371/journal.pone.0115701
Isoproterenol acts as a biased agonist of the alpha-1A-adrenoceptor that selectively activates the MAPK/ERK pathway
Abstract
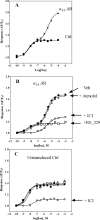
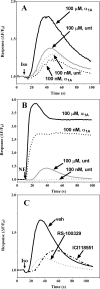
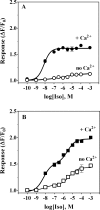
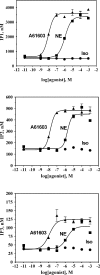
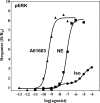
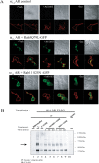
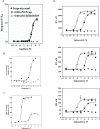
The α1A-AR is thought to couple predominantly to the Gαq/PLC pathway and lead to phosphoinositide hydrolysis and calcium mobilization, although certain agonists acting at this receptor have been reported to trigger activation of arachidonic acid formation and MAPK pathways. For several G protein-coupled receptors (GPCRs) agonists can manifest a bias for activation of particular effector signaling output, i.e., not all agonists of a given GPCR generate responses through utilization of the same signaling cascade(s). Previous work with Gαq coupling-defective variants of α1A-AR, as well as a combination of Ca2+ channel blockers, uncovered cross-talk between α1A-AR and β2-AR that leads to potentiation of a Gαq-independent signaling cascade in response to α1A-AR activation. We hypothesized that molecules exist that act as biased agonists to selectively activate this pathway. In this report, isoproterenol (Iso), typically viewed as β-AR-selective agonist, was examined with respect to activation of α1A-AR. α1A-AR selective antagonists were used to specifically block Iso evoked signaling in different cellular backgrounds and confirm its action at α1A-AR. Iso induced signaling at α1A-AR was further interrogated by probing steps along the Gαq /PLC, Gαs and MAPK/ERK pathways. In HEK-293/EBNA cells transiently transduced with α1A-AR, and CHO_α1A-AR stable cells, Iso evoked low potency ERK activity as well as Ca2+ mobilization that could be blocked by α1A-AR selective antagonists. The kinetics of Iso induced Ca2+ transients differed from typical Gαq- mediated Ca2+ mobilization, lacking both the fast IP3R mediated response and the sustained phase of Ca2+ re-entry. Moreover, no inositol phosphate (IP) accumulation could be detected in either cell line after stimulation with Iso, but activation was accompanied by receptor internalization. Data are presented that indicate that Iso represents a novel type of α1A-AR partial agonist with signaling bias toward MAPK/ERK signaling cascade that is likely independent of coupling to Gαq.
Conflict of interest statement
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

