Regulation of neuronal morphogenesis and positioning by ubiquitin-specific proteases in the cerebellum
- PMID: 25607801
- PMCID: PMC4301861
- DOI: 10.1371/journal.pone.0117076
Regulation of neuronal morphogenesis and positioning by ubiquitin-specific proteases in the cerebellum
Erratum in
-
Correction: Regulation of Neuronal Morphogenesis and Positioning by Ubiquitin-Specific Proteases in the Cerebellum.PLoS One. 2015 Jul 20;10(7):e0133943. doi: 10.1371/journal.pone.0133943. eCollection 2015. PLoS One. 2015. PMID: 26191798 Free PMC article. No abstract available.
-
Correction: Correction: Regulation of Neuronal Morphogenesis and Positioning by Ubiquitin-Specific Proteases in the Cerebellum.PLoS One. 2015 Aug 5;10(8):e0135535. doi: 10.1371/journal.pone.0135535. eCollection 2015. PLoS One. 2015. PMID: 26244976 Free PMC article. No abstract available.
Abstract
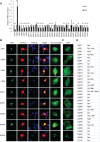
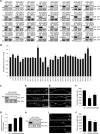
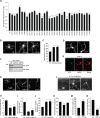
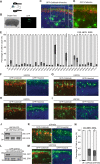
Ubiquitin signaling mechanisms play fundamental roles in the cell-intrinsic control of neuronal morphogenesis and connectivity in the brain. However, whereas specific ubiquitin ligases have been implicated in key steps of neural circuit assembly, the roles of ubiquitin-specific proteases (USPs) in the establishment of neuronal connectivity have remained unexplored. Here, we report a comprehensive analysis of USP family members in granule neuron morphogenesis and positioning in the rodent cerebellum. We identify a set of 32 USPs that are expressed in granule neurons. We also characterize the subcellular localization of the 32 USPs in granule neurons using a library of expression plasmids encoding GFP-USPs. In RNAi screens of the 32 neuronally expressed USPs, we uncover novel functions for USP1, USP4, and USP20 in the morphogenesis of granule neuron dendrites and axons and we identify a requirement for USP30 and USP33 in granule neuron migration in the rodent cerebellar cortex in vivo. These studies reveal that specific USPs with distinct spatial localizations harbor key functions in the control of neuronal morphogenesis and positioning in the mammalian cerebellum, with important implications for our understanding of the cell-intrinsic mechanisms that govern neural circuit assembly in the brain.
Conflict of interest statement
Figures
References
-
- Altman J, Bayer SA (1997) Development of the cerebellar system: in relation to its evolution, structure, and functions (Boca Raton: CRC Press; ).
-
- Hatten ME, Heintz N (1995) Mechanisms of neural patterning and specification in the developing cerebellum. Annual Rev Neurosci 18:385–408. - PubMed
-
- Hamori J, Somogyi J (1983) Differentiation of cerebellar mossy fiber synapses in the rat: a quantitative electron microscope study. Journal Comp Neurol 220: 365–377. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

