Neonatal ghrelin programs development of hypothalamic feeding circuits
- PMID: 25607843
- PMCID: PMC4319433
- DOI: 10.1172/JCI73688
Neonatal ghrelin programs development of hypothalamic feeding circuits
Abstract
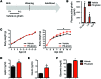
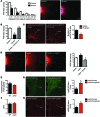
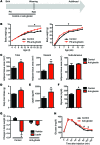
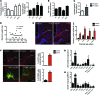
A complex neural network regulates body weight and energy balance, and dysfunction in the communication between the gut and this neural network is associated with metabolic diseases, such as obesity. The stomach-derived hormone ghrelin stimulates appetite through interactions with neurons in the arcuate nucleus of the hypothalamus (ARH). Here, we evaluated the physiological and neurobiological contribution of ghrelin during development by specifically blocking ghrelin action during early postnatal development in mice. Ghrelin blockade in neonatal mice resulted in enhanced ARH neural projections and long-term metabolic effects, including increased body weight, visceral fat, and blood glucose levels and decreased leptin sensitivity. In addition, chronic administration of ghrelin during postnatal life impaired the normal development of ARH projections and caused metabolic dysfunction. Consistent with these observations, direct exposure of postnatal ARH neuronal explants to ghrelin blunted axonal growth and blocked the neurotrophic effect of the adipocyte-derived hormone leptin. Moreover, chronic ghrelin exposure in neonatal mice also attenuated leptin-induced STAT3 signaling in ARH neurons. Collectively, these data reveal that ghrelin plays an inhibitory role in the development of hypothalamic neural circuits and suggest that proper expression of ghrelin during neonatal life is pivotal for lifelong metabolic regulation.
Figures


Comment in
-
Ghrelin and hypothalamic development: too little and too much of a good thing.J Clin Invest. 2015 Feb;125(2):490-2. doi: 10.1172/JCI79187. Epub 2015 Jan 20. J Clin Invest. 2015. PMID: 25607837 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

