CRALBP supports the mammalian retinal visual cycle and cone vision
- PMID: 25607845
- PMCID: PMC4319437
- DOI: 10.1172/JCI79651
CRALBP supports the mammalian retinal visual cycle and cone vision
Abstract
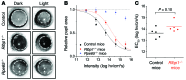
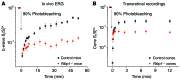
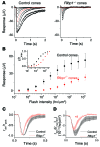
Mutations in the cellular retinaldehyde-binding protein (CRALBP, encoded by RLBP1) can lead to severe cone photoreceptor-mediated vision loss in patients. It is not known how CRALBP supports cone function or how altered CRALBP leads to cone dysfunction. Here, we determined that deletion of Rlbp1 in mice impairs the retinal visual cycle. Mice lacking CRALBP exhibited M-opsin mislocalization, M-cone loss, and impaired cone-driven visual behavior and light responses. Additionally, M-cone dark adaptation was largely suppressed in CRALBP-deficient animals. While rearing CRALBP-deficient mice in the dark prevented the deterioration of cone function, it did not rescue cone dark adaptation. Adeno-associated virus-mediated restoration of CRALBP expression specifically in Müller cells, but not retinal pigment epithelial (RPE) cells, rescued the retinal visual cycle and M-cone sensitivity in knockout mice. Our results identify Müller cell CRALBP as a key component of the retinal visual cycle and demonstrate that this pathway is important for maintaining normal cone-driven vision and accelerating cone dark adaptation.
Figures





References
Publication types
MeSH terms
Substances
Grants and funding
- EY002687/EY/NEI NIH HHS/United States
- R01 EY018826/EY/NEI NIH HHS/United States
- T32 GM098218/GM/NIGMS NIH HHS/United States
- T32 EY013934/EY/NEI NIH HHS/United States
- 5T32EY013360-15/EY/NEI NIH HHS/United States
- HG006346/HG/NHGRI NIH HHS/United States
- EY18826/EY/NEI NIH HHS/United States
- EY021126/EY/NEI NIH HHS/United States
- R21 HG006346/HG/NHGRI NIH HHS/United States
- GM076430-09/GM/NIGMS NIH HHS/United States
- R24 EY021126/EY/NEI NIH HHS/United States
- R01 GM076430/GM/NIGMS NIH HHS/United States
- P30 EY002687/EY/NEI NIH HHS/United States
- HG006790/HG/NHGRI NIH HHS/United States
- R01 EY019312/EY/NEI NIH HHS/United States
- T32 EY013360/EY/NEI NIH HHS/United States
- EY019312/EY/NEI NIH HHS/United States
- R01 HG006790/HG/NHGRI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

