Helical defects in microRNA influence protein binding by TAR RNA binding protein
- PMID: 25608000
- PMCID: PMC4301919
- DOI: 10.1371/journal.pone.0116749
Helical defects in microRNA influence protein binding by TAR RNA binding protein
Abstract
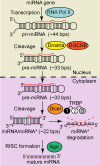
Background: MicroRNAs (miRNAs) are critical post-transcriptional regulators of gene expression. Their precursors have a globally A-form helical geometry, which prevents most proteins from identifying their nucleotide sequence. This suggests the hypothesis that local structural features (e.g., bulges, internal loops) play a central role in specific double-stranded RNA (dsRNA) selection from cellular RNA pools by dsRNA binding domain (dsRBD) containing proteins. Furthermore, the processing enzymes in the miRNA maturation pathway require tandem-dsRBD cofactor proteins for optimal function, suggesting that dsRBDs play a key role in the molecular mechanism for precise positioning of the RNA within these multi-protein complexes. Here, we focus on the tandem-dsRBDs of TRBP, which have been shown to bind dsRNA tightly.
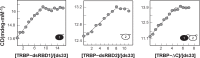
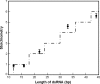
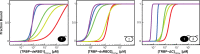
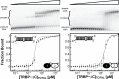
Methodology/principal findings: We present a combination of dsRNA binding assays demonstrating that TRBP binds dsRNA in an RNA-length dependent manner. Moreover, circular dichroism data shows that the number of dsRBD moieties bound to RNA at saturation is different for a tandem-dsRBD construct than for constructs with only one dsRBD per polypeptide, revealing another reason for the selective pressure to maintain multiple domains within a polypeptide chain. Finally, we show that helical defects in precursor miRNA alter the apparent dsRNA size, demonstrating that imperfections in RNA structure influence the strength of TRBP binding.
Conclusion/significance: We conclude that TRBP is responsible for recognizing structural imperfections in miRNA precursors, in the sense that TRBP is unable to bind imperfections efficiently and thus is positioned around them. We propose that once positioned around structural defects, TRBP assists Dicer and the rest of the RNA-induced silencing complex (RISC) in providing efficient and homogenous conversion of substrate precursor miRNA into mature miRNA downstream.
Conflict of interest statement
Figures




References
-
- Lee RC, Feinbaum RL, Ambros V (1993) The C. elegans heterochronic gene lin-4 encodes small RNAs with antisense complementarity to lin-14. Cell 75: 843–854. - PubMed
-
- Krol J, Loedige I, Filipowicz W (2010) The widespread regulation of microRNA biogenesis, function and decay. Nat Rev Genet 11: 597–610. - PubMed
-
- Kim VN, Han J, Siomi MC (2009) Biogenesis of small RNAs in animals. Nat Rev Mol Cell Biol 10: 126–139. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

