De novo isolation of antibodies with pH-dependent binding properties
- PMID: 25608219
- PMCID: PMC4623423
- DOI: 10.1080/19420862.2015.1006993
De novo isolation of antibodies with pH-dependent binding properties
Abstract
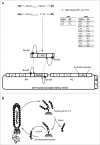
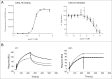
pH-dependent antibodies are engineered to release their target at a slightly acidic pH, a property making them suitable for clinical as well as biotechnological applications. Such antibodies were previously obtained by histidine scanning of pre-existing antibodies, a labor-intensive strategy resulting in antibodies that displayed residual binding to their target at pH 6.0. We report here the de novo isolation of pH-dependent antibodies selected by phage display from libraries enriched in histidines. Strongly pH-dependent clones with various affinity profiles against CXCL10 were isolated by this method. Our best candidate has nanomolar affinity for CXCL10 at pH 7.2, but no residual binding was detected at pH 6.0. We therefore propose that this new process is an efficient strategy to generate pH-dependent antibodies.
Keywords: BLI, bio-layer interferometry; CDR, complementary determining region; CDRH, CDR of the heavy chain; CDRL, CDR of the light chain; ELISA, enzyme-linked immunosorbent assay; GPCR, G protein-coupled receptor; KB, kinetic buffer; PBS, phosphate buffered saline; SPR, surface plasmon resonance; antibody recycling; chemokine; histidine; mAb, monoclonal antibody; monoclonal antibody; pH-dependency; phage display; phage libraries; scFv, single-chain variable fragment.
Figures


References
-
- Yagami H, Kato H, Tsumoto K, Tomita M. Monoclonal antibodies based on hybridoma technology. Pharm Pat Anal 2013; 2:249-63; http://dx.doi.org/10.4155/ppa.13.2 - DOI - PubMed
-
- Hairul Bahara NH, Tye GJ, Choong YS, Ong EB, Ismail A, Lim TS. Phage display antibodies for diagnostic applications. Biologicals 2013; 41:209-16; PMID:23647952; http://dx.doi.org/10.1016/j.biologicals.2013.04.001 - DOI - PubMed
-
- Vincent KJ, Zurini M. Current strategies in antibody engineering: Fc engineering and pH-dependent antigen binding, bispecific antibodies and antibody drug conjugates. Biotechnol J 2012; 7:1444-50; PMID:23125076; http://dx.doi.org/10.1002/biot.201200250 - DOI - PubMed
-
- Sela-Culang I, Kunik V, Ofran Y. The structural basis of antibody-antigen recognition. Front Immunol 2013; 4:302; PMID:24115948; http://dx.doi.org/10.3389/fimmu.2013.00302 - DOI - PMC - PubMed
-
- Aggarwal RS. What's fueling the biotech engine-2012 to 2013. Nat Biotechnol 2014; 32:32-39; PMID:24406926; http://dx.doi.org/10.1038/nbt.2794 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
