Pre-gestational vs gestational exposure to maternal obesity differentially programs the offspring in mice
- PMID: 25608625
- PMCID: PMC4452998
- DOI: 10.1007/s00125-014-3466-7
Pre-gestational vs gestational exposure to maternal obesity differentially programs the offspring in mice
Abstract
Aims/hypothesis: Maternal obesity is associated with an increased risk of obesity and impaired glucose homeostasis in offspring. However, it is not known whether a gestational or pre-gestational exposure confers similar risks, and if so, what the underlying mechanisms are.
Methods: We used reciprocal two-cell embryo transfers between mice fed either a control or high-fat diet (HFD) starting at the time of weaning. Gene expression in placenta was assessed by microarray analyses.
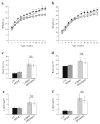
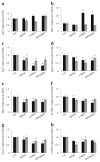
Results: A pre-gestational exposure to a maternal HFD (HFD/control) impaired fetal and placental growth despite a normal gestational milieu. Expression of imprinted genes and genes regulating vasculogenesis and lipid metabolism was markedly altered in placenta of HFD/control. An exposure to an HFD (control/HFD) only during gestation also resulted in fetal growth restriction and decreased placental weight. Interestingly, only a gestational exposure to an HFD (control/HFD) resulted in obesity and impaired glucose tolerance in adulthood.
Conclusions/interpretation: An HFD during pregnancy has profound consequences for the offspring later in life. Our data demonstrate that the mechanism underlying this phenomenon is not related to placental dysfunction, intrauterine growth restriction or postnatal weight gain, but rather an inability of the progeny to adapt to the abnormal gestational milieu of an HFD. Thus, the ability to adapt to an adverse intrauterine environment is conferred prior to pregnancy and it is possible that the effects of a maternal HFD may be transmitted to subsequent generations.
Conflict of interest statement
Figures




References
-
- Dietz WH. Overweight in childhood and adolescence. N Engl J Med. 2004;350:855–857. - PubMed
-
- Catalano PM. Obesity and pregnancy—the propagation of a viscous cycle? J Clin Endocrinol Metab. 2003;88:3505–3506. - PubMed
-
- Levin BE, Dunn-Meynell AA. Maternal obesity alters adiposity and monoamine function in genetically predisposed offspring. Am J Physiol Regul Integr Comp Physiol. 2002;283:R1087–R1093. - PubMed
-
- Levin BE, Govek E. Gestational obesity accentuates obesity in obesity-prone progeny. Am J Physiol. 1998;275:R1374–R1379. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

