ThermoTRPs and Pain
- PMID: 25608689
- PMCID: PMC4510032
- DOI: 10.1177/1073858414567884
ThermoTRPs and Pain
Abstract
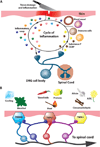
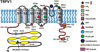
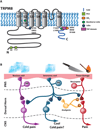
The ability of the body to perceive noxious stimuli lies in a heterogeneous group of primary somatosensory neurons termed nociceptors. The molecular receptors of noxious mechanical, temperature, or chemical stimuli are expressed in these neurons and have drawn considerable attention as possible targets for analgesic development to improve treatment for the millions who suffer from chronic pain conditions. A number of thermoTRPs, a subset of the transient receptor potential family of ion channels, are activated by a wide range on noxious stimuli. In this review, we review the function of these channels and examine the evidence that thermoTRPs play a vital role in acute, inflammatory and neuropathic nociception.
Keywords: TRPA1; TRPM3; TRPM8; TRPV1; TRPV3; TRPV4; analgesia; nociception; pain; thermoTRPs.
© The Author(s) 2015.
Conflict of interest statement
Conflict of Interest: The authors declare no competing financial interests
Figures
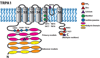
References
-
- Abe J, Hosokawa H, Sawada Y, Matsumura K, Kobayashi S. Ca2+-dependent PKC activation mediates menthol-induced desensitization of transient receptor potential M8. Neurosci. Lett. 2006;397:140–144. - PubMed
-
- Akopian AN, Ruparel NB, Jeske NA, Hargreaves KM. TRPA1 Desensitization in Sensory Neurons is Agonist- Dependent and Regulated by TRPV1-Directed Internalization. J Physiol [Internet] 2007 Available from: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dop.... - PMC - PubMed
-
- Alessandri-Haber N, Joseph E, Dina OA, Liedtke W, Levine JD. TRPV4 mediates pain-related behavior induced by mild hypertonic stimuli in the presence of inflammatory mediator. Pain. 2005;118:70–79. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

