In vitro expansion of corneal endothelial cells on biomimetic substrates
- PMID: 25609008
- PMCID: PMC4302312
- DOI: 10.1038/srep07955
In vitro expansion of corneal endothelial cells on biomimetic substrates
Abstract
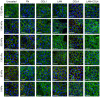
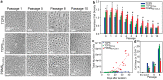
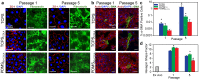
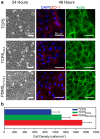
Corneal endothelial (CE) cells do not divide in vivo, leading to edema, corneal clouding and vision loss when the density drops below a critical level. The endothelium can be replaced by transplanting allogeneic tissue; however, access to donated tissue is limited worldwide resulting in critical need for new sources of corneal grafts. In vitro expansion of CE cells is a potential solution, but is challenging due to limited proliferation and loss of phenotype in vitro via endothelial to mesenchymal transformation (EMT) and senescence. We hypothesized that a bioengineered substrate recapitulating chemo-mechanical properties of Descemet's membrane would improve the in vitro expansion of CE cells while maintaining phenotype. Results show that bovine CE cells cultured on a polydimethylsiloxane surface with elastic modulus of 50 kPa and collagen IV coating achieved >3000-fold expansion. Cells grew in higher-density monolayers with polygonal morphology and ZO-1 localization at cell-cell junctions in contrast to control cells on polystyrene that lost these phenotypic markers coupled with increased α-smooth muscle actin expression and fibronectin fibril assembly. In total, these results demonstrate that a biomimetic substrate presenting native basement membrane ECM proteins and mechanical environment may be a key element in bioengineering functional CE layers for potential therapeutic applications.
Conflict of interest statement
Yes, there is potential competing interest. Drs. Palchesko, Funderburgh and Feinberg are co-inventors on a filed patent application related to the expansion technology in this manuscript.
Figures

References
-
- Zhu C. & Joyce N. C. Proliferative response of corneal endothelial cells from young and older donors. Invest Ophth Vis Sci. 45, 1743–1751 (2004). - PubMed
-
- Joyce N. C. Proliferative capacity of the corneal endothelium. Prog Retin Eye Res. 22, 359–389 (2003). - PubMed
-
- Joyce N. C., Meklir B., Joyce S. J. & Zieske J. D. Cell cycle protein expression and proliferative status in human corneal cells. Invest Ophth Vis Sci. 37, 645–655 (1996). - PubMed
-
- Joyce N. C., Navon S. E., Roy S. & Zieske J. D. Expression of cell cycle-associated proteins in human and rabbit corneal endothelium in situ. Invest Ophth Vis Sci. 37, 1566–1575 (1996). - PubMed
-
- Murphy C., Alvarado J., Juster R. & Maglio M. Prenatal and Postnatal Cellularity of the Human Corneal Endothelium - a Quantitative Histologic-Study. Invest Ophth Vis Sci. 25, 312–322 (1984). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

