The Efficacy of the Wee1 Inhibitor MK-1775 Combined with Temozolomide Is Limited by Heterogeneous Distribution across the Blood-Brain Barrier in Glioblastoma
- PMID: 25609063
- PMCID: PMC4401631
- DOI: 10.1158/1078-0432.CCR-14-2588
The Efficacy of the Wee1 Inhibitor MK-1775 Combined with Temozolomide Is Limited by Heterogeneous Distribution across the Blood-Brain Barrier in Glioblastoma
Abstract
Purpose: Wee1 regulates key DNA damage checkpoints, and in this study, the efficacy of the Wee1 inhibitor MK-1775 was evaluated in glioblastoma multiforme (GBM) xenograft models alone and in combination with radiation and/or temozolomide.
Experimental design: In vitro MK-1775 efficacy alone and in combination with temozolomide, and the impact on DNA damage, was analyzed by Western blotting and γH2AX foci formation. In vivo efficacy was evaluated in orthotopic and heterotopic xenografts. Drug distribution was assessed by conventional mass spectrometry (MS) and matrix-assisted laser desorption/ionization (MALDI)-MS imaging.
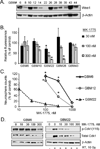
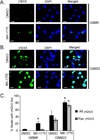
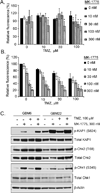
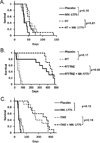
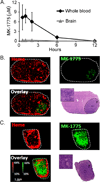
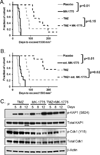
Results: GBM22 (IC50 = 68 nmol/L) was significantly more sensitive to MK-1775 compared with five other GBM xenograft lines, including GBM6 (IC50 >300 nmol/L), and this was associated with a significant difference in pan-nuclear γH2AX staining between treated GBM22 (81% cells positive) and GBM6 (20% cells positive) cells. However, there was no sensitizing effect of MK-1775 when combined with temozolomide in vitro. In an orthotopic GBM22 model, MK-1775 was ineffective when combined with temozolomide, whereas in a flank model of GBM22, MK-1775 exhibited both single-agent and combinatorial activity with temozolomide. Consistent with limited drug delivery into orthotopic tumors, the normal brain to whole blood ratio following a single MK-1775 dose was 5%, and MALDI-MS imaging demonstrated heterogeneous and markedly lower MK-1775 distribution in orthotopic as compared with heterotopic GBM22 tumors.
Conclusions: Limited distribution to brain tumors may limit the efficacy of MK-1775 in GBM.
©2015 American Association for Cancer Research.
Conflict of interest statement
Figures
References
-
- Smith J, Tho LM, Xu N, Gillespie DA. The ATM-Chk2 and ATR-Chk1 pathways in DNA damage signaling and cancer. Adv Cancer Res. 2010;108:73–112. - PubMed
-
- Malumbres M, Barbacid M. Cell cycle, CDKs and cancer: a changing paradigm. Nature Reviews Cancer. 2009;9:153–166. - PubMed
-
- Satyanarayana A, Kaldis P. Mammalian cell-cycle regulation: several Cdks, numerous cyclins and diverse compensatory mechanisms. Oncogene. 2009;28:2925–2939. - PubMed
-
- Hirai H, Arai T, Okada M, Nishibata T, Kobayashi M, Sakai N, et al. MK-1775, a small molecule Wee1 inhibitor, enhances anti-tumor efficacy of various DNA-damaging agents, including 5-fluorouracil. Cancer Biology & Therapy. 2010;9:514–522. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

