Nitric oxide donors increase PVR/CD155 DNAM-1 ligand expression in multiple myeloma cells: role of DNA damage response activation
- PMID: 25609078
- PMCID: PMC4311457
- DOI: 10.1186/s12885-015-1023-5
Nitric oxide donors increase PVR/CD155 DNAM-1 ligand expression in multiple myeloma cells: role of DNA damage response activation
Abstract
Background: DNAX accessory molecule-1 (DNAM-1) is an activating receptor constitutively expressed by macrophages/dendritic cells and by T lymphocytes and Natural Killer (NK) cells, having an important role in anticancer responses; in this regard, combination therapies able to enhance the expression of DNAM-1 ligands on tumor cells are of therapeutic interest. In this study, we investigated the effect of different nitric oxide (NO) donors on the expression of the DNAM-1 ligand Poliovirus Receptor/CD155 (PVR/CD155) in multiple myeloma (MM) cells.
Methods: Six MM cell lines, SKO-007(J3), U266, OPM-2, RPMI-8226, ARK and LP1 were used to investigate the activity of different nitric oxide donors [DETA-NO and the NO-releasing prodrugs NCX4040 (NO-aspirin) and JS-K] on the expression of PVR/CD155, using Flow Cytometry and Real-Time PCR. Western-blot and specific inhibitors were employed to investigate the role of soluble guanylyl cyclase/cGMP and activation of the DNA damage response (DDR).
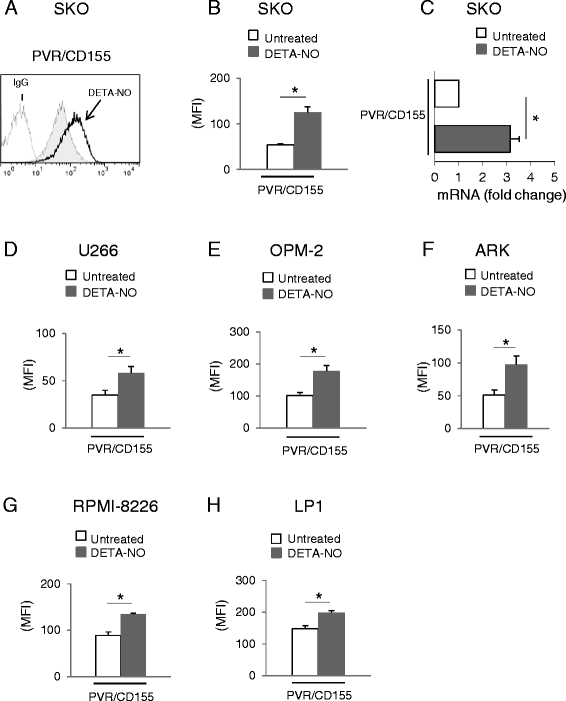
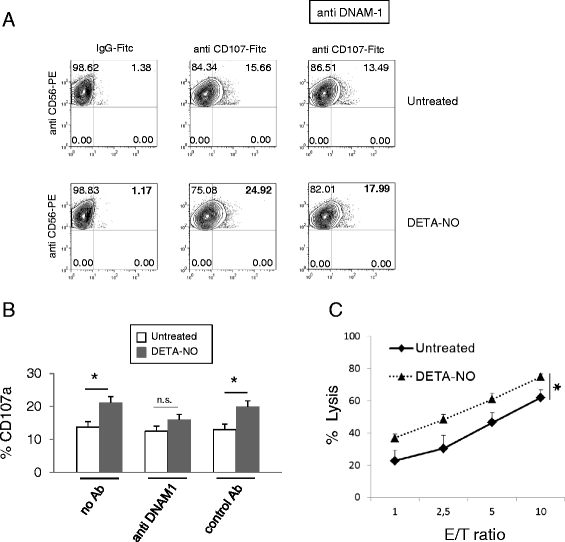
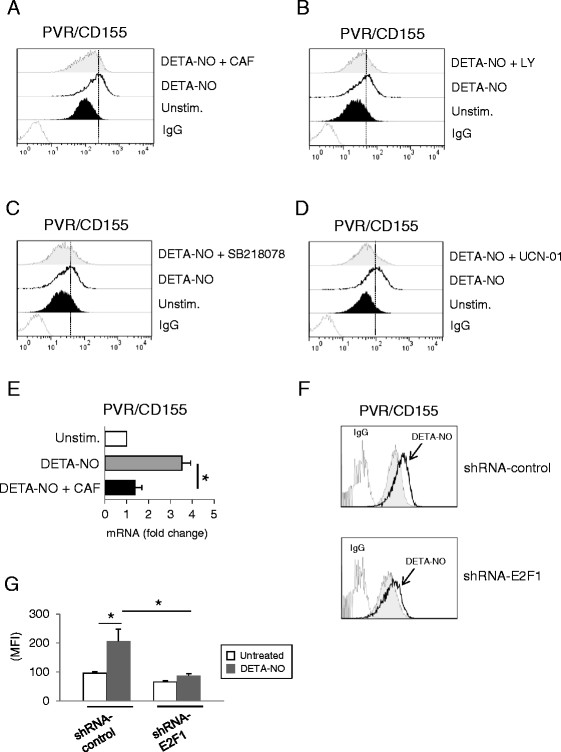
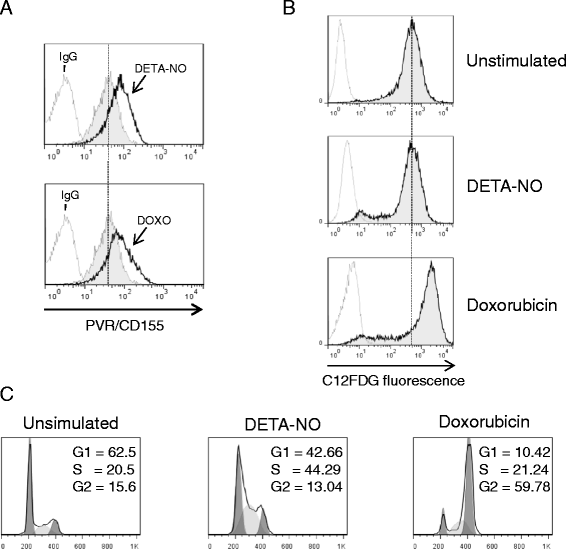
Results: Our results indicate that increased levels of nitric oxide can upregulate PVR/CD155 cell surface and mRNA expression in MM cells; in addition, exposure to nitric oxide donors renders myeloma cells more efficient to activate NK cell degranulation and enhances their ability to trigger NK cell-mediated cytotoxicity. We found that activation of the soluble guanylyl cyclase and increased cGMP concentrations by nitric oxide is not involved in the up-regulation of ligand expression. On the contrary, treatment of MM cells with nitric oxide donors correlated with the activation of a DNA damage response pathway and inhibition of the ATM /ATR/Chk1/2 kinase activities by specific inhibitors significantly abrogates up-regulation.
Conclusions: The present study provides evidence that regulation of the PVR/CD155 DNAM-1 ligand expression by nitric oxide may represent an additional immune-mediated mechanism and supports the anti-myeloma activity of nitric oxide donors.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

