Rab5-family guanine nucleotide exchange factors bind retromer and promote its recruitment to endosomes
- PMID: 25609093
- PMCID: PMC4357511
- DOI: 10.1091/mbc.E14-08-1281
Rab5-family guanine nucleotide exchange factors bind retromer and promote its recruitment to endosomes
Erratum in
-
Correction.Mol Biol Cell. 2015 Apr 1;26(7):1411. Mol Biol Cell. 2015. PMID: 25823929 Free PMC article. No abstract available.
Abstract
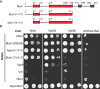
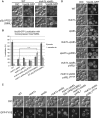
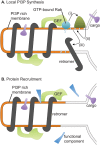
The retromer complex facilitates the sorting of integral membrane proteins from the endosome to the late Golgi. In mammalian cells, the efficient recruitment of retromer to endosomes requires the lipid phosphatidylinositol 3-phosphate (PI3P) as well as Rab5 and Rab7 GTPases. However, in yeast, the role of Rabs in recruiting retromer to endosomes is less clear. We identified novel physical interactions between retromer and the Saccharomyces cerevisiae VPS9-domain Rab5-family guanine nucleotide exchange factors (GEFs) Muk1 and Vps9. Furthermore, we identified a new yeast VPS9 domain-containing protein, VARP-like 1 (Vrl1), which is related to the human VARP protein. All three VPS9 domain-containing proteins show localization to endosomes, and the presence of any one of them is necessary for the endosomal recruitment of retromer. We find that expression of an active VPS9-domain protein is required for correct localization of the phosphatidylinositol 3-kinase Vps34 and the production of endosomal PI3P. These results suggest that VPS9 GEFs promote retromer recruitment by establishing PI3P-enriched domains at the endosomal membrane. The interaction of retromer with distinct VPS9 GEFs could thus link GEF-dependent regulatory inputs to the temporal or spatial coordination of retromer assembly or function.
© 2015 Bean et al. This article is distributed by The American Society for Cell Biology under license from the author(s). Two months after publication it is available to the public under an Attribution–Noncommercial–Share Alike 3.0 Unported Creative Commons License (http://creativecommons.org/licenses/by-nc-sa/3.0).
Figures




References
-
- Attar N, Cullen PJ. The retromer complex. Adv Enzyme Regul. 2010;50:216–236. - PubMed
-
- Babu M, Vlasblom J, Pu S, Guo X, Graham C, Bean BDM, Burston HE, Vizeacoumar FJ, Snider J, Phanse S, et al. Interaction landscape of membrane-protein complexes in Saccharomyces cerevisiae. Nature. 2012;489:585–589. - PubMed
-
- Balderhaar H, Arlt H, Ostrowicz C, Brocker C, Sundermann F, Brandt R, Babst M, Ungermann C. The Rab GTPase Ypt7 is linked to retromer-mediated receptor recycling and fusion at the yeast late endosome. J Cell Sci. 2010;123:4085–4094. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

