Surface-enhanced resonance Raman scattering nanostars for high-precision cancer imaging
- PMID: 25609167
- PMCID: PMC4414254
- DOI: 10.1126/scitranslmed.3010633
Surface-enhanced resonance Raman scattering nanostars for high-precision cancer imaging
Abstract
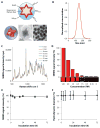
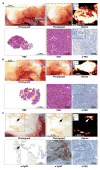
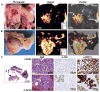
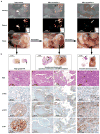
The inability to visualize the true extent of cancers represents a significant challenge in many areas of oncology. The margins of most cancer types are not well demarcated because the cancer diffusely infiltrates the surrounding tissues. Furthermore, cancers may be multifocal and characterized by the presence of microscopic satellite lesions. Such microscopic foci represent a major reason for persistence of cancer, local recurrences, and metastatic spread, and are usually impossible to visualize with currently available imaging technologies. An imaging method to reveal the true extent of tumors is desired clinically and surgically. We show the precise visualization of tumor margins, microscopic tumor invasion, and multifocal locoregional tumor spread using a new generation of surface-enhanced resonance Raman scattering (SERRS) nanoparticles, which are termed SERRS nanostars. The SERRS nanostars feature a star-shaped gold core, a Raman reporter resonant in the near-infrared spectrum, and a primer-free silication method. In genetically engineered mouse models of pancreatic cancer, breast cancer, prostate cancer, and sarcoma, and in one human sarcoma xenograft model, SERRS nanostars enabled accurate detection of macroscopic malignant lesions, as well as microscopic disease, without the need for a targeting moiety. Moreover, the sensitivity (1.5 fM limit of detection) of SERRS nanostars allowed imaging of premalignant lesions of pancreatic and prostatic neoplasias. High sensitivity and broad applicability, in conjunction with their inert gold-silica composition, render SERRS nanostars a promising imaging agent for more precise cancer imaging and resection.
Copyright © 2015, American Association for the Advancement of Science.
Conflict of interest statement
Figures


Comment in
-
Highlights from the latest articles in nanomedicine for deep tumor imaging and phototherapy.Nanomedicine (Lond). 2015;10(11):1681-3. doi: 10.2217/nnm.15.63. Nanomedicine (Lond). 2015. PMID: 26080692 No abstract available.
References
-
- Kircher MF, Willmann JK. Molecular body imaging: MR imaging, CT, and US. Part II. Applications. Radiology. 2012;264:349–368. - PubMed
Publication types
MeSH terms
Grants and funding
- K08 DA016396/DA/NIDA NIH HHS/United States
- R01 EB017748/EB/NIBIB NIH HHS/United States
- P30CA013696/CA/NCI NIH HHS/United States
- P30 CA008748/CA/NCI NIH HHS/United States
- K08 CA163961/CA/NCI NIH HHS/United States
- P01 CA013106/CA/NCI NIH HHS/United States
- T32 GM007739/GM/NIGMS NIH HHS/United States
- R25 CA096945/CA/NCI NIH HHS/United States
- DRR-29-14/DRCRF/Damon Runyon Cancer Research Foundation/United States
- R01 CA157980/CA/NCI NIH HHS/United States
- P30 CA013696/CA/NCI NIH HHS/United States
- 2R25-CA096945/CA/NCI NIH HHS/United States
- P50 CA140146/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

