A phase 3 randomized, double-blind, placebo-controlled trial of ganitumab or placebo in combination with gemcitabine as first-line therapy for metastatic adenocarcinoma of the pancreas: the GAMMA trial
- PMID: 25609246
- PMCID: PMC4804122
- DOI: 10.1093/annonc/mdv027
A phase 3 randomized, double-blind, placebo-controlled trial of ganitumab or placebo in combination with gemcitabine as first-line therapy for metastatic adenocarcinoma of the pancreas: the GAMMA trial
Abstract
Background: This double-blind, phase 3 study assessed the efficacy and safety of ganitumab combined with gemcitabine as first-line treatment of metastatic pancreatic cancer.
Patients and methods: Patients with previously untreated metastatic pancreatic adenocarcinoma were randomly assigned 2 : 2 : 1 to receive intravenous gemcitabine 1000 mg/m(2) (days 1, 8, and 15 of each 28-day cycle) plus placebo, ganitumab 12 mg/kg, or ganitumab 20 mg/kg (days 1 and 15 of each cycle). The primary end point was overall survival (OS). Secondary end points included progression-free survival (PFS), safety, and efficacy by levels of circulating biomarkers.
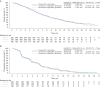
Results: Overall, 322 patients were randomly assigned to placebo, 318 to ganitumab 12 mg/kg, and 160 to ganitumab 20 mg/kg. The study was stopped based on results from a preplanned futility analysis; the final results are reported. Median OS was 7.2 months [95% confidence interval (CI), 6.3-8.2] in the placebo arm, 7.0 months (95% CI, 6.2-8.5) in the ganitumab 12-mg/kg arm [hazard ratio (HR), 1.00; 95% CI, 0.82-1.21; P = 0.494], and 7.1 months (95% CI, 6.4-8.5) in the ganitumab 20-mg/kg arm (HR, 0.97; 95% CI, 0.76-1.23; P = 0.397). Median PFS was 3.7, 3.6 (HR, 1.00; 95% CI, 0.84-1.20; P = 0.520), and 3.7 months (HR, 0.97; 95% CI, 0.77-1.22; P = 0.403), respectively. No unexpected toxicity was observed with ganitumab plus gemcitabine. The circulating biomarkers assessed [insulin-like growth factor-1 (IGF-1), IGF-binding protein-2, and -3] were not associated with a treatment effect on OS or PFS by ganitumab.
Conclusion: Ganitumab combined with gemcitabine had manageable toxicity but did not improve OS, compared with gemcitabine alone in unselected patients with metastatic pancreatic cancer.
Clinical trial registration: ClinicalTrials.gov NCT01231347.
Keywords: IGF-1 receptor; biomarker; ganitumab; gemcitabine; pancreatic cancer.
© The Author 2015. Published by Oxford University Press on behalf of the European Society for Medical Oncology. All rights reserved. For permissions, please email: journals.permissions@oup.com.
Figures
References
-
- SEER Stat Fact Sheets: Pancreas Cancer. National Cancer Institute; http://seer.cancer.gov/statfacts/html/pancreas.html (3 March 2014, date last accessed).
-
- Baxter NN, Whitson BA, Tuttle TM. Trends in the treatment and outcome of pancreatic cancer in the United States. Ann Surg Oncol 2007; 14: 1320–1326. - PubMed
-
- Tempero M, Plunkett W, Ruiz Van Haperen V, et al. Randomized phase II comparison of dose-intense gemcitabine: thirty-minute infusion and fixed dose rate infusion in patients with pancreatic adenocarcinoma. J Clin Oncol 2003; 21: 3402–3408. - PubMed
-
- NCCN Clinical Practice Guidelines in Oncology: Pancreatic Adenocarcinoma v.1.2014. Fort Washington, PA: National Comprehensive Cancer Network, 2014.
-
- Seufferlein T, Bachet JB, Van Cutsem E, Rouqier P, ESMO Guidelines Working Group. Pancreatic adenocarcinoma: ESMO-ESDO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2012; 23(Suppl 7): vii33–vii40. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

