Hyperactivity of newborn Pten knock-out neurons results from increased excitatory synaptic drive
- PMID: 25609613
- PMCID: PMC4300333
- DOI: 10.1523/JNEUROSCI.3144-14.2015
Hyperactivity of newborn Pten knock-out neurons results from increased excitatory synaptic drive
Abstract
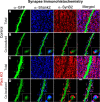
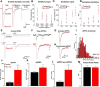
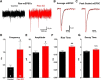
Developing neurons must regulate morphology, intrinsic excitability, and synaptogenesis to form neural circuits. When these processes go awry, disorders, including autism spectrum disorder (ASD) or epilepsy, may result. The phosphatase Pten is mutated in some patients having ASD and seizures, suggesting that its mutation disrupts neurological function in part through increasing neuronal activity. Supporting this idea, neuronal knock-out of Pten in mice can cause macrocephaly, behavioral changes similar to ASD, and seizures. However, the mechanisms through which excitability is enhanced following Pten depletion are unclear. Previous studies have separately shown that Pten-depleted neurons can drive seizures, receive elevated excitatory synaptic input, and have abnormal dendrites. We therefore tested the hypothesis that developing Pten-depleted neurons are hyperactive due to increased excitatory synaptogenesis using electrophysiology, calcium imaging, morphological analyses, and modeling. This was accomplished by coinjecting retroviruses to either "birthdate" or birthdate and knock-out Pten in granule neurons of the murine neonatal dentate gyrus. We found that Pten knock-out neurons, despite a rapid onset of hypertrophy, were more active in vivo. Pten knock-out neurons fired at more hyperpolarized membrane potentials, displayed greater peak spike rates, and were more sensitive to depolarizing synaptic input. The increased sensitivity of Pten knock-out neurons was due, in part, to a higher density of synapses located more proximal to the soma. We determined that increased synaptic drive was sufficient to drive hypertrophic Pten knock-out neurons beyond their altered action potential threshold. Thus, our work contributes a developmental mechanism for the increased activity of Pten-depleted neurons.
Keywords: Pten; autism spectrum disorder; dendritic spine; neuron structure function; seizure; synaptogenesis.
Copyright © 2015 the authors 0270-6474/15/350943-17$15.00/0.
Figures






References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous
