Contributions of diverse excitatory and inhibitory neurons to recurrent network activity in cerebral cortex
- PMID: 25609625
- PMCID: PMC4300319
- DOI: 10.1523/JNEUROSCI.2279-14.2015
Contributions of diverse excitatory and inhibitory neurons to recurrent network activity in cerebral cortex
Abstract
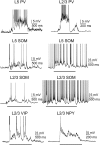
The recurrent synaptic architecture of neocortex allows for self-generated network activity. One form of such activity is the Up state, in which neurons transiently receive barrages of excitatory and inhibitory synaptic inputs that depolarize many neurons to spike threshold before returning to a relatively quiescent Down state. The extent to which different cell types participate in Up states is still unclear. Inhibitory interneurons have particularly diverse intrinsic properties and synaptic connections with the local network, suggesting that different interneurons might play different roles in activated network states. We have studied the firing, subthreshold behavior, and synaptic conductances of identified cell types during Up and Down states in layers 5 and 2/3 in mouse barrel cortex in vitro. We recorded from pyramidal cells and interneurons expressing parvalbumin (PV), somatostatin (SOM), vasoactive intestinal peptide (VIP), or neuropeptide Y. PV cells were the most active interneuron subtype during the Up state, yet the other subtypes also received substantial synaptic conductances and often generated spikes. In all cell types except PV cells, the beginning of the Up state was dominated by synaptic inhibition, which decreased thereafter; excitation was more persistent, suggesting that inhibition is not the dominant force in terminating Up states. Compared with barrel cortex, SOM and VIP cells were much less active in entorhinal cortex during Up states. Our results provide a measure of functional connectivity of various neuron types in barrel cortex and suggest differential roles for interneuron types in the generation and control of persistent network activity.
Keywords: Up state; barrel cortex; entorhinal cortex; inhibition; interneuron; slice.
Copyright © 2015 the authors 0270-6474/15/351089-17$15.00/0.
Figures










References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
