Synaptic consolidation: from synapses to behavioral modeling
- PMID: 25609644
- PMCID: PMC6605543
- DOI: 10.1523/JNEUROSCI.3989-14.2015
Synaptic consolidation: from synapses to behavioral modeling
Abstract
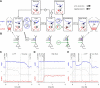
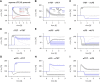
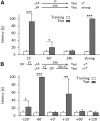
Synaptic plasticity, a key process for memory formation, manifests itself across different time scales ranging from a few seconds for plasticity induction up to hours or even years for consolidation and memory retention. We developed a three-layered model of synaptic consolidation that accounts for data across a large range of experimental conditions. Consolidation occurs in the model through the interaction of the synaptic efficacy with a scaffolding variable by a read-write process mediated by a tagging-related variable. Plasticity-inducing stimuli modify the efficacy, but the state of tag and scaffold can only change if a write protection mechanism is overcome. Our model makes a link from depotentiation protocols in vitro to behavioral results regarding the influence of novelty on inhibitory avoidance memory in rats.
Keywords: consolidation; modeling; synaptic tagging.
Copyright © 2015 the authors 0270-6474/15/351319-16$15.00/0.
Figures




References
-
- Amit D, Fusi S. Learning in neural networks with material synapses. Neural Computing. 1994;6:957–982. doi: 10.1162/neco.1994.6.5.957. - DOI
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
