Subnuclear domain proteins in cancer cells support the functions of RUNX2 in the DNA damage response
- PMID: 25609707
- PMCID: PMC4327387
- DOI: 10.1242/jcs.160051
Subnuclear domain proteins in cancer cells support the functions of RUNX2 in the DNA damage response
Abstract
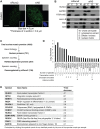
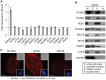
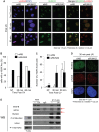
Cancer cells exhibit modifications in nuclear architecture and transcriptional control. Tumor growth and metastasis are supported by RUNX family transcriptional scaffolding proteins, which mediate the assembly of nuclear-matrix-associated gene-regulatory hubs. We used proteomic analysis to identify RUNX2-dependent protein-protein interactions associated with the nuclear matrix in bone, breast and prostate tumor cell types and found that RUNX2 interacts with three distinct proteins that respond to DNA damage - RUVBL2, INTS3 and BAZ1B. Subnuclear foci containing these proteins change in intensity or number following UV irradiation. Furthermore, RUNX2, INTS3 and BAZ1B form UV-responsive complexes with the serine-139-phosphorylated isoform of H2AX (γH2AX). UV irradiation increases the interaction of BAZ1B with γH2AX and decreases histone H3 lysine 9 acetylation levels, which mark accessible chromatin. RUNX2 depletion prevents the BAZ1B-γH2AX interaction and attenuates loss of H3K9 and H3K56 acetylation. Our data are consistent with a model in which RUNX2 forms functional complexes with BAZ1B, RUVBL2 and INTS3 to mount an integrated response to DNA damage. This proposed cytoprotective function for RUNX2 in cancer cells might clarify its expression in chemotherapy-resistant and/or metastatic tumors.
Keywords: BAZ1B; Breast; Cancer; DNA damage response; INTS3; Nuclear matrix; Osteosarcoma; Prostate; Proteomics; RUNX2.
© 2015. Published by The Company of Biologists Ltd.
Figures





References
-
- Albrethsen J., Knol J. C., Piersma S. R., Pham T. V., de Wit M., Mongera S., Carvalho B., Verheul H. M. W., Fijneman R. J. A., Meijer G. A. et al. (2010). Subnuclear proteomics in colorectal cancer: identification of proteins enriched in the nuclear matrix fraction and regulation in adenoma to carcinoma progression. Mol. Cell Proteomics 9, 988–1005 10.1074/mcp.M900546-MCP200 - DOI - PMC - PubMed
-
- Barboro P., D'Arrigo C., Repaci E., Bagnasco L., Orecchia P., Carnemolla B., Patrone E., Balbi C. (2009). Proteomic analysis of the nuclear matrix in the early stages of rat liver carcinogenesis: identification of differentially expressed and MAR-binding proteins. Exp. Cell Res. 315, 226–239 10.1016/j.yexcr.2008.10.017 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

