Neutrophil IL-1β processing induced by pneumolysin is mediated by the NLRP3/ASC inflammasome and caspase-1 activation and is dependent on K+ efflux
- PMID: 25609842
- PMCID: PMC4369676
- DOI: 10.4049/jimmunol.1401624
Neutrophil IL-1β processing induced by pneumolysin is mediated by the NLRP3/ASC inflammasome and caspase-1 activation and is dependent on K+ efflux
Abstract
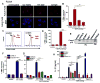
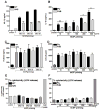
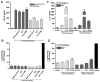
Although neutrophils are the most abundant cells in acute infection and inflammation, relatively little attention has been paid to their role in inflammasome formation and IL-1β processing. In the present study, we investigated the mechanism by which neutrophils process IL-1β in response to Streptococcus pneumoniae. Using a murine model of S. pneumoniae corneal infection, we demonstrated a requirement for IL-1β in bacterial clearance, and we showed that Nod-like receptor protein 3 (NLRP3), apoptosis-associated speck-like protein containing a caspase activation and recruitment domain (ASC), and caspase-1 are essential for IL-1β production and bacterial killing in the cornea. Neutrophils in infected corneas had multiple specks with enzymatically active caspase-1 (YVAD-FLICA 660), and bone marrow neutrophils stimulated with heat-killed S. pneumoniae (signal 1) and pneumolysin (signal 2) exhibited multiple specks when stained for NLRP3, ASC, or Caspase-1. High-molecular mass ASC complexes were also detected, consistent with oligomer formation. Pneumolysin induced K(+) efflux in neutrophils, and blocking K(+) efflux inhibited caspase-1 activation and IL-1β processing; however, neutrophils did not undergo pyroptosis, indicating that K(+) efflux and IL-1β processing is not a consequence of cell death. There was also no role for lysosomal destabilization or neutrophil elastase in pneumolysin-mediated IL-1β processing in neutrophils. Taken together, these findings demonstrate an essential role for neutrophil-derived IL-1β in S. pneumoniae infection, and they elucidate the role of the NLRP3 inflammasome in cleavage and secretion of IL-1β in neutrophils. Given the ubiquitous presence of neutrophils in acute bacterial and fungal infections, these findings will have implications for other microbial diseases.
Copyright © 2015 by The American Association of Immunologists, Inc.
Figures





References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

