Letter to the editor: naltrexone sustained-release/bupropion sustained-release for the management of obesity: review of the data to date
- PMID: 25609921
- PMCID: PMC4293294
- DOI: 10.2147/DDDT.S77881
Letter to the editor: naltrexone sustained-release/bupropion sustained-release for the management of obesity: review of the data to date
Figures


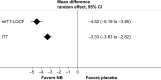
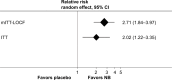
Comment on
-
Naltrexone sustained-release/bupropion sustained-release for the management of obesity: review of the data to date.Drug Des Devel Ther. 2014 Sep 18;8:1419-27. doi: 10.2147/DDDT.S55587. eCollection 2014. Drug Des Devel Ther. 2014. PMID: 25258511 Free PMC article.
References
-
- Gray LJ, Cooper N, Dunkley A, et al. A systematic review and mixed treatment comparison of pharmacological interventions for the treatment of obesity. Obes Rev. 2012;13(6):483–498. - PubMed
-
- James WP, Caterson ID, Coutinho W, et al. SCOUT Investigators Effect of sibutramine on cardiovascular outcomes in overweight and obese subjects. N Engl J Med. 2010;363(10):905–917. - PubMed
-
- Hollander P, Gupta AK, Plodkowski R, et al. COR-Diabetes Study Group Effects of naltrexone sustained-release/bupropion sustained-release combination therapy on body weight and glycemic parameters in overweight and obese patients with type 2 diabetes. Diabetes Care. 2013;36(12):4022–4029. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

