Preparation, in vitro and in vivo evaluation of polymeric nanoparticles based on hyaluronic acid-poly(butyl cyanoacrylate) and D-alpha-tocopheryl polyethylene glycol 1000 succinate for tumor-targeted delivery of morin hydrate
- PMID: 25609946
- PMCID: PMC4293365
- DOI: 10.2147/IJN.S73971
Preparation, in vitro and in vivo evaluation of polymeric nanoparticles based on hyaluronic acid-poly(butyl cyanoacrylate) and D-alpha-tocopheryl polyethylene glycol 1000 succinate for tumor-targeted delivery of morin hydrate
Abstract
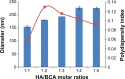
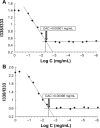
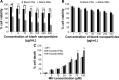
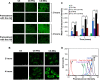
Herein, we describe the preparation of a targeted cellular delivery system for morin hydrate (MH), based on a low-molecular-weight hyaluronic acid-poly(butyl cyanoacrylate) (HA-PBCA) block copolymer. In order to enhance the therapeutic effect of MH, D-alpha-tocopheryl polyethylene glycol 1000 succinate (TPGS) was mixed with HA-PBCA during the preparation process. The MH-loaded HA-PBCA "plain" nanoparticle (MH-PNs) and HA-PBCA/TPGS "mixed" nanoparticles (MH-MNs) were concomitantly characterized in terms of loading efficiency, particle size, zeta potential, critical aggregation concentration, and morphology. The obtained MH-PNs and MH-MNs exhibited a spherical morphology with a negative zeta potential and a particle size less than 200 nm, favorable for drug targeting. Remarkably, the addition of TPGS resulted in about 1.6-fold increase in drug-loading. The in vitro cell viability experiment revealed that MH-MNs enhanced the cytotoxicity of MH in A549 cells compared with MH solution and MH-PNs. Furthermore, blank MNs containing TPGS exhibited selective cytotoxic effects against cancer cells without diminishing the viability of normal cells. In addition, the cellular uptake study indicated that MNs resulted in 2.28-fold higher cellular uptake than that of PNs, in A549 cells. The CD44 receptor competitive inhibition and the internalization pathway studies suggested that the internalization mechanism of the nanoparticles was mediated mainly by the CD44 receptors through a clathrin-dependent endocytic pathway. More importantly, MH-MNs exhibited a higher in vivo antitumor potency and induced more tumor cell apoptosis than did MH-PNs, following intravenous administration to S180 tumor-bearing mice. Overall, the results imply that the developed nanoparticles are promising vehicles for the targeted delivery of lipophilic anticancer drugs.
Keywords: TPGS; anti-tumor effect; hyaluronic acid; morin hydrate; nanoparticles.
Figures



References
-
- Gopal JV. Morin Hydrate: Botanical origin, pharmacological activity and its applications: A mini-review. Pharmacognosy Journal. 2013;5(3):123–126.
-
- Sivaramakrishnan V, Devaraj SN. Morin fosters apoptosis in experimental hepatocellular carcinogenesis model. Chem Biol Interact. 2010;183(2):284–292. - PubMed
-
- Romero I, Páez A, Ferruelo A, Luján M, Berenguer A. Polyphenols in red wine inhibit the proliferation and induce apoptosis of LNCaP cells. BJU Int. 2002;89(9):950–954. - PubMed
-
- Zhang S, Yang X, Morris ME. Flavonoids are inhibitors of breast cancer resistance protein (ABCG2)-mediated transport. Mol Pharmacol. 2004;65(5):1208–1216. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

