Revealing neuronal function through microelectrode array recordings
- PMID: 25610364
- PMCID: PMC4285113
- DOI: 10.3389/fnins.2014.00423
Revealing neuronal function through microelectrode array recordings
Abstract
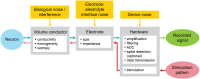
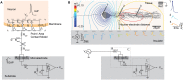
Microelectrode arrays and microprobes have been widely utilized to measure neuronal activity, both in vitro and in vivo. The key advantage is the capability to record and stimulate neurons at multiple sites simultaneously. However, unlike the single-cell or single-channel resolution of intracellular recording, microelectrodes detect signals from all possible sources around every sensor. Here, we review the current understanding of microelectrode signals and the techniques for analyzing them. We introduce the ongoing advancements in microelectrode technology, with focus on achieving higher resolution and quality of recordings by means of monolithic integration with on-chip circuitry. We show how recent advanced microelectrode array measurement methods facilitate the understanding of single neurons as well as network function.
Keywords: CMOS; extracellular recording; microelectrode array; multi-scale modeling; multielectrode array; neuron-electrode interface; neuronal function; stimulation.
Figures











References
-
- Abeles M., Gerstein G. L. (1988). Detecting spatiotemporal firing patterns among simultaneously recorded single neurons. J. Neurophysiol. 60, 909–24. - PubMed
-
- Anastassiou C. A., Buzsáki G., Koch C. (2013). Biophysics of extracellular spikes, in Principles of Neural Coding, eds. Quiroga R., Panzeri S. (Boca Raton, FL: CRC Press; ), 15–36 10.1201/b14756-4 - DOI
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

