Neurodevelopmental origin and adult neurogenesis of the neuroendocrine hypothalamus
- PMID: 25610370
- PMCID: PMC4285089
- DOI: 10.3389/fncel.2014.00440
Neurodevelopmental origin and adult neurogenesis of the neuroendocrine hypothalamus
Abstract
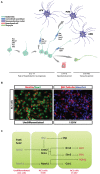
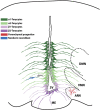
The adult hypothalamus regulates many physiological functions and homeostatic loops, including growth, feeding and reproduction. In mammals, the hypothalamus derives from the ventral diencephalon where two distinct ventricular proliferative zones have been described. Although a set of transcription factors regulating the hypothalamic development has been identified, the exact molecular mechanisms that drive the differentiation of hypothalamic neural precursor cells (NPCs) toward specific neuroendocrine neuronal subtypes is yet not fully disclosed. Neurogenesis has been also reported in the adult hypothalamus at the level of specific niches located in the ventrolateral region of ventricle wall, where NPCs have been identified as radial glia-like tanycytes. Here we review the molecular and cellular systems proposed to support the neurogenic potential of developing and adult hypothalamic NPCs. We also report new insights on the mechanisms by which adult hypothalamic neurogenesis modulates key functions of this brain region. Finally, we discuss how environmental factors may modulate the adult hypothalamic neurogenic cascade.
Keywords: adult stem cells; hypothalamus; neural progenitor cells; neural stem cell (NSC); neurogenesis; tanycytes.
Figures
Similar articles
-
Neural Progenitor Cells and the Hypothalamus.Cells. 2023 Jul 11;12(14):1822. doi: 10.3390/cells12141822. Cells. 2023. PMID: 37508487 Free PMC article. Review.
-
Irx3 and Irx5 - Novel Regulatory Factors of Postnatal Hypothalamic Neurogenesis.Front Neurosci. 2021 Nov 2;15:763856. doi: 10.3389/fnins.2021.763856. eCollection 2021. Front Neurosci. 2021. PMID: 34795556 Free PMC article. Review.
-
Hypothalamic Neurogenesis as an Adaptive Metabolic Mechanism.Front Neurosci. 2017 Apr 5;11:190. doi: 10.3389/fnins.2017.00190. eCollection 2017. Front Neurosci. 2017. PMID: 28424582 Free PMC article. Review.
-
Neurogenesis in the adult hypothalamus: A distinct form of structural plasticity involved in metabolic and circadian regulation, with potential relevance for human pathophysiology.Handb Clin Neurol. 2021;179:125-140. doi: 10.1016/B978-0-12-819975-6.00006-6. Handb Clin Neurol. 2021. PMID: 34225958 Review.
-
Hypothalamic tanycytes-masters and servants of metabolic, neuroendocrine, and neurogenic functions.Front Neurosci. 2015 Oct 29;9:387. doi: 10.3389/fnins.2015.00387. eCollection 2015. Front Neurosci. 2015. PMID: 26578855 Free PMC article. Review.
Cited by
-
Exploring the Role of Neuroplasticity in Development, Aging, and Neurodegeneration.Brain Sci. 2023 Nov 21;13(12):1610. doi: 10.3390/brainsci13121610. Brain Sci. 2023. PMID: 38137058 Free PMC article. Review.
-
Proliferative Markers and Neural Stem Cells Markers in Tanycytes of the Third Cerebral Ventricle in Rats.Bull Exp Biol Med. 2023 Feb;174(4):564-570. doi: 10.1007/s10517-023-05748-8. Epub 2023 Mar 10. Bull Exp Biol Med. 2023. PMID: 36894817
-
Environmental Enrichment, Age, and PPARα Interact to Regulate Proliferation in Neurogenic Niches.Front Neurosci. 2016 Mar 9;10:89. doi: 10.3389/fnins.2016.00089. eCollection 2016. Front Neurosci. 2016. PMID: 27013951 Free PMC article.
-
An Efficient Method for Generating Murine Hypothalamic Neurospheres for the Study of Regional Neural Progenitor Biology.Endocrinology. 2020 Apr 1;161(4):bqaa035. doi: 10.1210/endocr/bqaa035. Endocrinology. 2020. PMID: 32154873 Free PMC article.
-
Therapeutic efficacy and promise of stem cell-derived extracellular vesicles in Alzheimer's disease and other aging-related disorders.Ageing Res Rev. 2023 Dec;92:102088. doi: 10.1016/j.arr.2023.102088. Epub 2023 Oct 11. Ageing Res Rev. 2023. PMID: 37827304 Free PMC article. Review.
References
-
- Acampora D., Postiglione M. P., Avantaggiato V., Di Bonito M., Vaccarino F. M., Michaud J., et al. . (1999). Progressive impairment of developing neuroendocrine cell lineages in the hypothalamus of mice lacking the Orthopedia gene. Genes Dev. 13, 2787–2800. 10.1101/gad.13.21.2787 - DOI - PMC - PubMed
-
- Altman J., Bayer S. A. (1986). The development of the rat hypothalamus. Adv. Anat. Embryol. Cell Biol. 100, 1–178. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

