Immunostimulatory activity of protein hydrolysate from oviductus ranae on macrophage in vitro
- PMID: 25610475
- PMCID: PMC4283414
- DOI: 10.1155/2014/180234
Immunostimulatory activity of protein hydrolysate from oviductus ranae on macrophage in vitro
Abstract
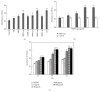
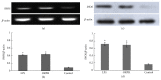
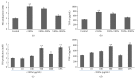
Oviductus Ranae is the dry oviduct of Rana chensinensis, which is also called R. chensinensis oil. Oviductus Ranae is a valuable Chinese crude drug and is recorded in the Pharmacopoeia of the People's Republic of China. The aim of this study was to investigate the immunostimulatory activity of protein hydrolysate of Oviductus Ranae (ORPH) and to assess its possible mechanism. Immunomodulatory activity of ORPH was examined in murine macrophage RAW 264.7 cells. The effect of ORPH on the phagocytic activity of macrophages was determined by the neutral red uptake assay. After treatment with ORPH, NO production levels in the culture supernatant were investigated by Griess assay. The mRNA and protein expressions of inducible nitric oxide synthase (iNOS) were detected by RT-PCR and Western blotting. The production of TNF-α, IL-1β, and IL-6 after treatment with ORPH was measured using ELISA assay. In addition, NF-κB levels were also investigated by Western blot. The results showed that ORPH enhanced the phagocytosis of macrophage, increased productions of TNF-α, IL-1β, IL-6, and NO in RAW 264.7 cells, and upregulated the mRNA and protein expression of iNOS. Besides, NF-κB, levels in RAW 264.7 cells were elevated after ORPH treatment. These findings suggested that ORPH might stimulate macrophage activities by activating the NF-κB pathway.
Figures




Similar articles
-
Investigation of the anti-glioma activity of Oviductus ranae protein hydrolysate.Biomed Pharmacother. 2016 Jul;81:176-181. doi: 10.1016/j.biopha.2016.04.015. Epub 2016 Apr 16. Biomed Pharmacother. 2016. PMID: 27261592
-
Hamayou () protein hydrolysate ameliorates depression by regulating the mitogen-activated protein kinase pathway.J Tradit Chin Med. 2025 Jun;45(3):493-507. doi: 10.19852/j.cnki.jtcm.2025.03.007. J Tradit Chin Med. 2025. PMID: 40524289 Free PMC article.
-
Oviductus Ranae protein hydrolyzate prevents menopausal osteoporosis by regulating TGFβ/BMP2 signaling.Arch Gynecol Obstet. 2019 Mar;299(3):873-882. doi: 10.1007/s00404-018-5033-9. Epub 2019 Jan 16. Arch Gynecol Obstet. 2019. PMID: 30649603
-
In vitro and in vivo immunostimulatory effects of hot water extracts from the leaves of Artemisia princeps Pampanini cv. Sajabal.J Ethnopharmacol. 2013 Aug 26;149(1):254-62. doi: 10.1016/j.jep.2013.06.030. Epub 2013 Jun 27. J Ethnopharmacol. 2013. PMID: 23810843
-
Anti-inflammatory effect of the six compounds isolated from Nauclea officinalis Pierrc ex Pitard, and molecular mechanism of strictosamide via suppressing the NF-κB and MAPK signaling pathway in LPS-induced RAW 264.7 macrophages.J Ethnopharmacol. 2017 Jan 20;196:66-74. doi: 10.1016/j.jep.2016.12.007. Epub 2016 Dec 15. J Ethnopharmacol. 2017. PMID: 27989509
Cited by
-
Acute Toxicity, Antioxidant, and Antifatigue Activities of Protein-Rich Extract from Oviductus ranae.Oxid Med Cell Longev. 2018 Feb 25;2018:9021371. doi: 10.1155/2018/9021371. eCollection 2018. Oxid Med Cell Longev. 2018. PMID: 29991975 Free PMC article.
-
Immunostimulatory Activity of Lactic Acid Bacteria Cell-Free Supernatants through the Activation of NF-κB and MAPK Signaling Pathways in RAW 264.7 Cells.Microorganisms. 2022 Nov 13;10(11):2247. doi: 10.3390/microorganisms10112247. Microorganisms. 2022. PMID: 36422317 Free PMC article.
-
Traditional Uses, Bioactive Constituents, Biological Functions, and Safety Properties of Oviductus ranae as Functional Foods in China.Oxid Med Cell Longev. 2019 Jun 2;2019:4739450. doi: 10.1155/2019/4739450. eCollection 2019. Oxid Med Cell Longev. 2019. PMID: 31281578 Free PMC article. Review.
-
Understanding immune-modulatory efficacy in vitro.Chem Biol Interact. 2022 Jan 25;352:109776. doi: 10.1016/j.cbi.2021.109776. Epub 2021 Dec 11. Chem Biol Interact. 2022. PMID: 34906553 Free PMC article. Review.
-
Purification and Characterization of a Novel Pentadecapeptide from Protein Hydrolysates of Cyclina sinensis and Its Immunomodulatory Effects on RAW264.7 Cells.Mar Drugs. 2019 Jan 6;17(1):30. doi: 10.3390/md17010030. Mar Drugs. 2019. PMID: 30621347 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources

