Xiao Yao San Improves Depressive-Like Behaviors in Rats with Chronic Immobilization Stress through Modulation of Locus Coeruleus-Norepinephrine System
- PMID: 25610478
- PMCID: PMC4291141
- DOI: 10.1155/2014/605914
Xiao Yao San Improves Depressive-Like Behaviors in Rats with Chronic Immobilization Stress through Modulation of Locus Coeruleus-Norepinephrine System
Abstract
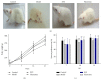
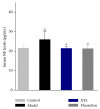
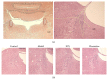
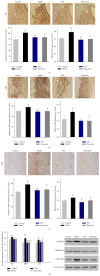
Most research focuses on the hypothalamic-pituitary-adrenal (HPA) axis, hypothalamus-pituitary-thyroid (HPT) axis, and hypothalamus-pituitary-gonadal (HPGA) axis systems of abnormalities of emotions and behaviors induced by stress, while no studies of Chinese herbal medicine such as Xiao Yao San (XYS) on the mechanisms of locus coeruleus-norepinephrine (LC-NE) system have been reported. Therefore, experiments were carried out to observe mechanism of LC-NE system in response to chronic immobilization stress (CIS) and explore the antidepressant effect of XYS. Rat model was established by CIS. LC morphology in rat was conducted. The serum norepinephrine (NE) concentrations and NE biosynthesis such as tyrosine hydroxylase (TH), dopamine-β-hydroxylase (DBH), and corticotrophin-releasing-factor (CRF) in LC were determined. Results showed that there were no discernible alterations in LC in rats. The serum NE concentrations, positive neurons, mean optical density (MOD), and protein levels of TH, DBH, and CRF in model group were significantly increased compared to the control group. But XYS-treated group displayed a significantly decreased in NE levels and expressions of TH, DBH, and CRF compared to the model group. In conclusion, CIS can activate LC-NE system to release NE and then result in a significant decrease in rats. XYS treatment can effectively improve depressive-like behaviors in rats through inhibition of LC-NE neurons activity.
Figures
Similar articles
-
Xiao Yao San Improves Depressive-Like Behavior in Rats through Modulation of β-Arrestin 2-Mediated Pathways in Hippocampus.Evid Based Complement Alternat Med. 2014;2014:902516. doi: 10.1155/2014/902516. Epub 2014 Jul 7. Evid Based Complement Alternat Med. 2014. PMID: 25097660 Free PMC article.
-
The use of neurotoxins to characterize the rates and subcellular distributions of axonally transported dopamine-beta-hydroxylase, tyrosine hydroxylase and norepinephrine in the rat brain.Brain Res. 1979 May 25;168(2):331-50. doi: 10.1016/0006-8993(79)90174-4. Brain Res. 1979. PMID: 87244
-
Hippocampal norepinephrine-like voltammetric responses following infusion of corticotropin-releasing factor into the locus coeruleus.Brain Res Bull. 2000 Mar 1;51(4):319-26. doi: 10.1016/s0361-9230(99)00241-5. Brain Res Bull. 2000. PMID: 10704782
-
Brain circuits involved in corticotropin-releasing factor-norepinephrine interactions during stress.Ann N Y Acad Sci. 2004 Jun;1018:25-34. doi: 10.1196/annals.1296.003. Ann N Y Acad Sci. 2004. PMID: 15240349 Review.
-
Aging is associated in the 344/N Fischer rat with decreased stress responsivity of central and peripheral catecholaminergic systems and impairment of the hypothalamic-pituitary-adrenal axis.Ann N Y Acad Sci. 1995 Dec 29;771:491-511. doi: 10.1111/j.1749-6632.1995.tb44705.x. Ann N Y Acad Sci. 1995. PMID: 8597425 Review.
Cited by
-
Xiaoyaosan prevents atherosclerotic vulnerable plaque formation through heat shock protein/glucocorticoid receptor axis-mediated mechanism.Am J Transl Res. 2019 Sep 15;11(9):5531-5545. eCollection 2019. Am J Transl Res. 2019. PMID: 31632527 Free PMC article.
-
Nine traditional Chinese herbal formulas for the treatment of depression: an ethnopharmacology, phytochemistry, and pharmacology review.Neuropsychiatr Dis Treat. 2016 Sep 20;12:2387-2402. doi: 10.2147/NDT.S114560. eCollection 2016. Neuropsychiatr Dis Treat. 2016. PMID: 27703356 Free PMC article. Review.
-
Influence of Xiaoyaosan on depressive-like behaviors in chronic stress-depressed rats through regulating tryptophan metabolism in hippocampus.Neuropsychiatr Dis Treat. 2018 Dec 18;15:21-31. doi: 10.2147/NDT.S185295. eCollection 2019. Neuropsychiatr Dis Treat. 2018. PMID: 30587994 Free PMC article.
-
Involvement of Hepatic SHIP2 and PI3K/Akt Signalling in the Regulation of Plasma Insulin by Xiaoyaosan in Chronic Immobilization-Stressed Rats.Molecules. 2019 Jan 29;24(3):480. doi: 10.3390/molecules24030480. Molecules. 2019. PMID: 30699999 Free PMC article.
-
Research progress on classical traditional chinese medicine formula xiaoyaosan in the treatment of depression.Front Pharmacol. 2022 Aug 4;13:925514. doi: 10.3389/fphar.2022.925514. eCollection 2022. Front Pharmacol. 2022. PMID: 35991880 Free PMC article. Review.
References
-
- Green M. K., Rani C. S. S., Joshi A., Soto-Piña A. E., Martinez P. A., Frazer A., Strong R., Morilak D. A. Prenatal stress induces long term stress vulnerability, compromising stress response systems in the brain and impairing extinction of conditioned fear after adult stress. Neuroscience. 2011;192:438–451. doi: 10.1016/j.neuroscience.2011.06.041. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

